Result: KI efficiency increased up to 30% compared to standard plasmid
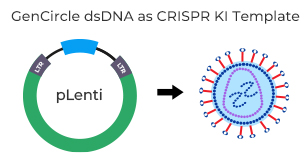
- CRISPR KI template( 2.5 kb insertion)
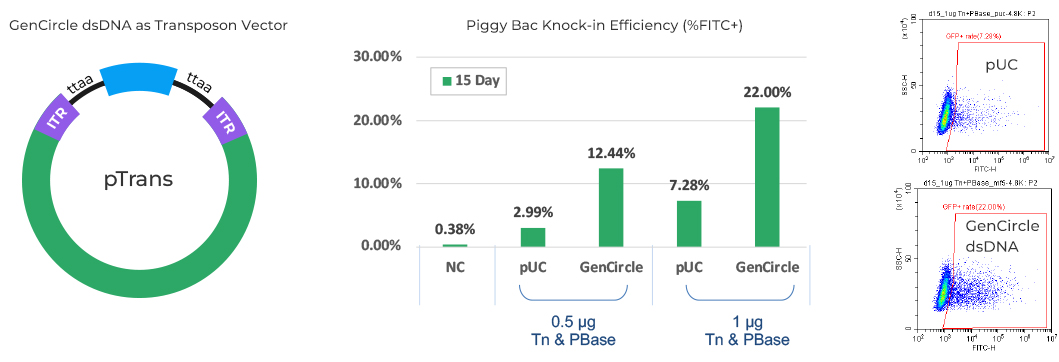
- Transposon vector for KI(4.8 kb insertion)

 IVD Raw Materials
IVD Raw Materials

| Catalog No. | Price(USD) | Quantity |
|---|---|---|
| SC1848 | $99.44 | 1 |
| SC1634 | $0 | 1 |
| SC1848 | $139.21 | 1 |
| SC1634-1 | TBD | 1 |
| SC1848 | $119.33 | 1 |
| » See More Items | ||
Home » CRISPR Services » Homology-Directed Repair (HDR) Knock-in Templates » GenCircle Double-Stranded DNA
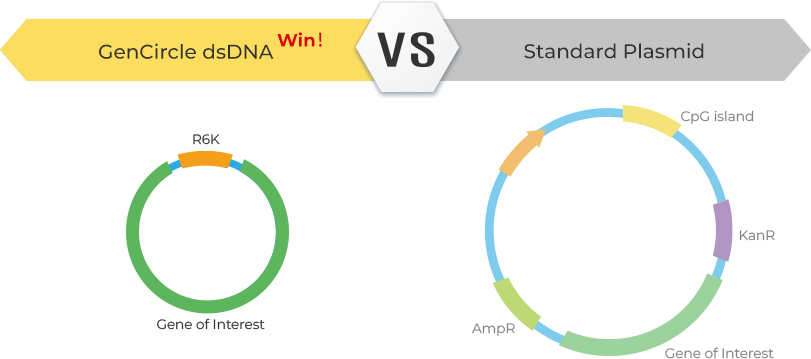
GenCircle™ dsDNA is a novel, small circular double-stranded DNA vector with a minimal 429 bp backbone, making it an ideal solution for a variety of applications, including non-viral knock-in templates for gene editing, viral packaging plasmids, non-viral gene-of-interest vectors, and antigen expression vectors. Unlike standard plasmid vectors, GenCircle™ dsDNA has no antibiotic resistance genes or bacterial origin sequences, ensuring it cannot replicate outside its engineered production host strain.
Unlike traditional plasmid vectors, which often have backbones larger than 1.5 kb and contain antibiotic resistance genes, GenCircle™ dsDNA eliminates these components, reducing concerns about drug resistance in in vivo applications. Its streamlined design offers superior stability, lower cytotoxicity, reduced immunogenicity, and higher transfection efficiency.
By customizing GenCircle™ dsDNA to carry your target gene, you can achieve safer and more effective outcomes for therapeutic development, vaccine production, and advanced genetic research.
Safer: No Antibiotic Resistance Genes
Simpler: Lower Cytotoxicity & Immunogenicity
Smaller: High-Fidelity 429 bp Backbone
More Efficient: Enhanced Performance Across Applications
| Service | Grade | GOI Length* | Quantity* | Format | TAT | Application |
|---|---|---|---|---|---|---|
| GenCircle™ dsDNA | HighPure | 1-20 kb | 100 μg - g | Freeze-dried powder/ Solution liquid (Buffer is optional) | From 11 Days |
|
| UltraPure | 1 mg - g |
|
* Listed are general ex vivo applications. UltraPure is recommended for in vivo studies and for challenging cell types, such as primary cells and stem cells.
Select off-the-shelf GenCircle™ dsDNA products are also available here.
Are you looking to validate your experimental system with GenCircle™ or optimize editing efficiency? Try our off-the-shelf GenCircle™ products:
| Cat. No. | Product Name | Quantity | Price | Order |
|---|---|---|---|---|
|
KIOK32
|
GenCircle™ dsDNA_PiggyBac_CMV_Luciferase_GFP |
|
$100.00 2 nmol |
|
| Test Specification/ Detection Method | Release Criteria | HighPure Grade | UltraPure Grade |
|---|---|---|---|
| Concentration by A260 | Guaranteed quantity | ||
| Identity by Sanger Sequencing | Correct sequence | ||
| Identity by Restriction Analysis | DNA bands of expected sizes detected by agarose gel electrophoresis (AGE) | ||
| Purity by A260/280 | 1.8-2.0 | ||
| Plasmid Topology by AGE | ≥ 90% supercoil | ||
| Microbial Limit | No growth within 72 hours | ||
| Endotoxin | ≤ 0.01 EU/μg by Gel Clot Test | ||
| ≤ 0.005 EU/μg by Chromogenic TAL Assay | |||
| Residual Host RNA by AGE | No bands detected | ||
| Residual Host DNA | Non-detectable by AGE Gray Scale Analysis (GIS=0) | ||
| ≤ 5% by qPCR | |||
| Residual Total Protein by NanoOrange | ≤1% | ||
| pH by pH-metry | TE Buffer: 8.00 ±0.50 | ||
| ddH2O: (5.00-7.00) ±0.50 | |||
| PBS: 7.40 ±0.50 | |||
| Tris: 7.88 ±0.50 |
Result: KI efficiency increased up to 30% compared to standard plasmid


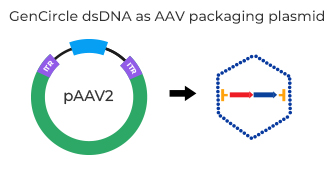
Result: Viral titer improve up to 3 fold compared to standard plasmid, with stable ITR.
| Name | Virus Titer (IFU/ml) |
Antibiotic resistance gene |
|---|---|---|
| pRRL-PGK-EGFP (Regular Plasmid) |
1.23E+8 |
Significant residue (Ct value<30) |
| pRRL-PGK-EGFP MF (GenCircle dsDNA) |
3.81E+8 |
Undetectable (Ct≥35 ) |
| Name | Virus Titer (IFU/ml) |
Antibiotic resistance gene |
|---|---|---|
| CMV EGFP AAV2 (Regular Plasmid) |
1.12E+12 | 2% |
| CMV EGFP MF (GenCircle dsDNA ) |
1.22E+12 | Undetectable |
Result: RNA transcription level increased up to 4 fold, with reduced immune response compared to standard plasmid.

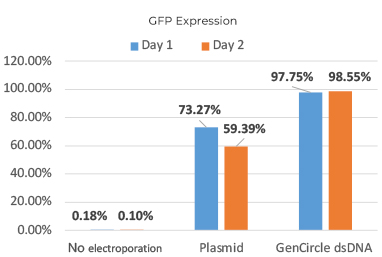
Result: protein expression level increased up to 39% compare to standard plasmid.
GenScript has successfully delivered multiple orders with a wide range of GOI lengths, including multiple complex or difficult-to-synthesize projects.
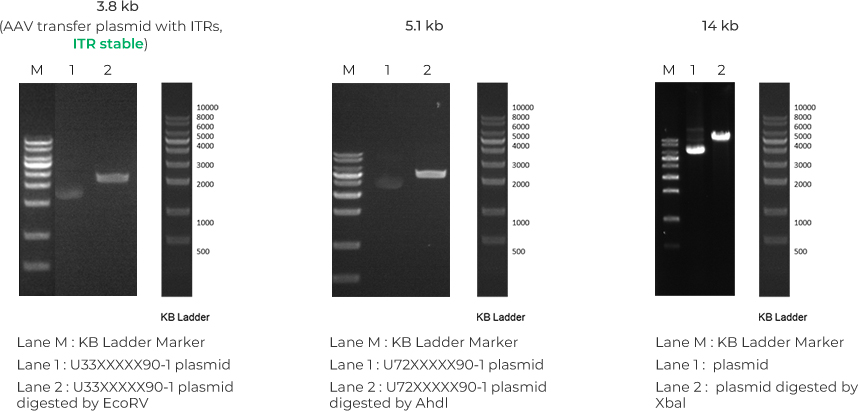
While we have only tested and delivered up to 14kb to date, we don’t foresee any limitations to delivering GenCircle dsDNA up to 50 kb in length. For any insert over 14 kb, IQR would be needed.
As we can deliver gene synthesis up to 200 kb, GenCircle dsDNA should be able to accommodate insert sequences up to 200 kb, following a technical evaluation.
The GenCircle dsDNA backbone does not carry any expression or integration elements, so all functional elements for downstream applications must be placed within the insert sequence (dsDNA sequence):
The production E.coli strain is transformed with a unique parental plasmid containing a kanamycin resistance gene selection marker, and GenCircle dsDNA is then generated in vivo. After transformation and selection, the parental plasmid is induced to self-cleave into one GenCircle and one redundant backbone circle. The redundant backbone circle cannot replicate and is quickly metabolized by the production E.coli cell. GenCircle replaces the residual parental plasmid due to the unique design incompatibility of the parental plasmid backbone.
Please see the workflow below to see which steps in the production process are antibiotic-free. Colony screening and negative selection for antibiotic resistance are performed prior to fermentation and maxi prep. We do not test for antibiotic residuals in the final product.
The R6K origin of replication allows GenCircle dsDNA to replicate itself in its specific host E.coli, but not in other bacteria that have not been engineered.
Yes, for Preclinical grade GenCircle orders, we offer an animal-free option at an extra charge. Additionally, customers may request an entirely animal-free production process (culture medium, enzyme, and reagent), with a provided TSE/BSE statement.
The recommended conditions for primary T cell knock-in with the Thermo Fisher Scientific Neon™ transfection system (10ul kit) are as follows:
This protocol was used by the GenScript R&D department in the knock-in case study (Case 1) above to achieve ~50% KI efficiency for a 2.5 kb insertion in the TRAC locus. We highly recommend that customers optimize conditions for their specific experiment and cell type.
When used for lentiviral packaging, GenCircle dsDNA is a 3rd generation lentivirus system, utilizing four plasmids. GenCircle dsDNA serves as the transfer plasmid encoding your insert of interest, plus three helper plasmids.
When used for AAV packaging, GenCircle dsDNA serves as the transfer plasmid encoding your insert of interest, plus two helper plasmids.
We can deliver GenCircle dsDNA in 2 ml EP tubes (default option) or 96 well plates, formatted as lyophilized powder or in a liquid solution (TE, ddH2O, Tris, PBS, or Nuclease-free water), at the concentration of your choice.
Yes. We own the IP for GenCircle™ dsDNA and the production process of GenCircle™ dsDNA, and has applied for the international patent under PCT. Customers can use GenCircle dsDNA without IP concerns in the RUO or commercialization stage.
We do not charge any licensing fees for RUO applications using GenCircle dsDNA.
For any questions regarding licensing fees for clinical or commercial use, please reach out to one of our CRISPR experts at crispr@genscript.com.
We provide two primary QC tests for our RUO grade GenCircle dsDNA templates:
Pre-clinical grade GenCircle dsDNA is also tested by qPCR and HCP ELISA.
After the GenCircle dsDNA product replaces the residual parental plasmid, the production E. Coli cells are screened until a single colony without antibiotic resistance is selected to be used as the seed for GenCircle fermentation. This proprietary purification process ensures complete removal of the parental plasmid.
Lyophilized GenCircle dsDNA is stable under -20°C for at least one year. Liquid products need to be stored at -20°C. Repeated freezing and thawing for either format should be avoided. If necessary, divide the stock solution into small aliquots and only thaw the amount needed for each test.
Generally, 1-2 μg GenCircle dsDNA are used per million cells. Further dose optimization is necessary for your specific use case.
Yes! We offer Knock-in Optimization Kits (KIOK) to help you effectively optimize your CAR insertion process with a pre-validated sequence, design, and protocol. By choosing this pre-designed kit, you can achieve up to 75% in cost savings compared to ordering customized synthesis services! Learn more here.
Yes, we offer a variety of protocols that include step-by-step instructions for HDR knock-ins with some of the most commonly used electroporators, including the Thermo Fisher Scientific Neon® Transfection System and MaxCyte® Electroporation Platform.
You can view and download them for free here.
