-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Products
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Online Shop
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![GenScript Life Science Research Fund GenScript Life Science Research Fund]()
GenScript Life Science
Research Grant ProgramFostering collaboration and excellence in life science research
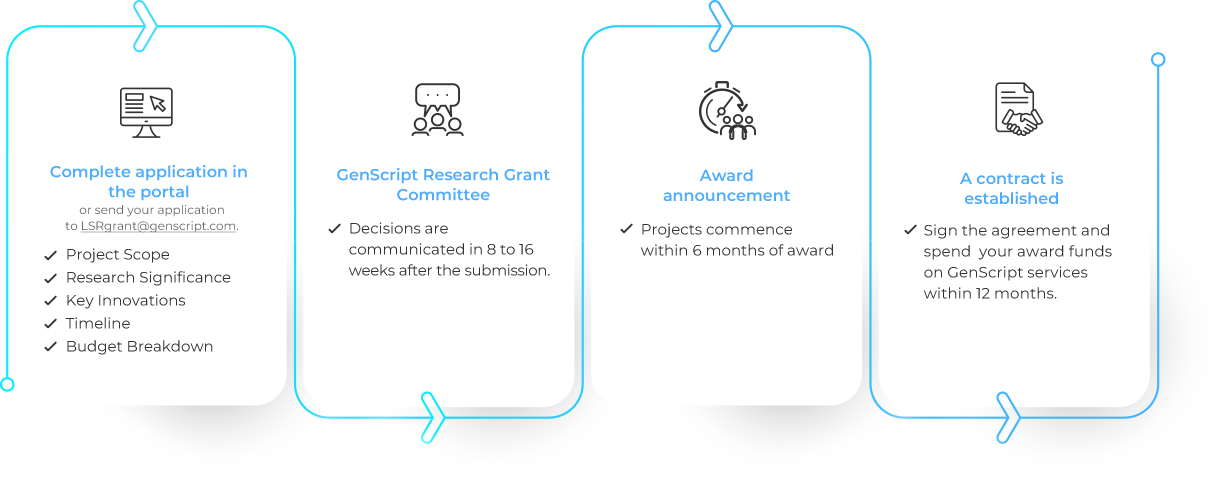
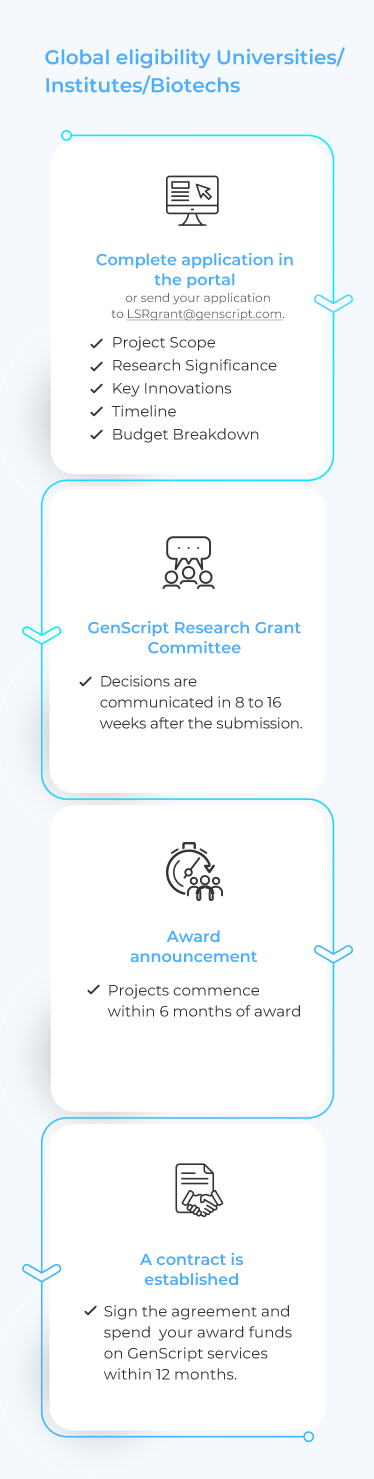
Apply NowWhat is it?
The GenScript Life Science Research Grant Program (LSRG Program), now in its second year, continues its mission to support breakthroughs in life science research. This initiative is dedicated to empowering researchers by providing grant funding explicitly earmarked for purchasing GenScript reagents and services. Researchers in diverse life science fields, including but not limited to those listed below, are encouraged to apply and benefit from this unique opportunity to advance their work.
 Gene and Cell
Therapy Development
Gene and Cell
Therapy Development
 Antibody Drug
Discovery
Antibody Drug
Discovery  AI Drug Discovery
AI Drug Discovery  Vaccine
Development
Vaccine
Development
 Diagnostics
Diagnostics How it works
GenScript provides comprehensive “one-stop” solutions for projects in the identified research areas through its advanced technologies and platforms. The Life Science Research Grant Program will leverage GenScript’s existing capabilities to accelerate projects by partnering with investigators globally on their proposed projects.

Over 50 grants are planned and will be awarded based on scientific merit, potential for impact, and strategic alignment with GenScript’s primary areas of scientific interest.

Grants will be awarded at a range of funding levels, dependent on GenScript’s internal committee assessment of the project’s budget needs. The maximum amount to be awarded is set at 100,000 USD for qualifying projects.

Projects are expected to commence promptly after receiving grant funding from GenScript, and research activities should generally take place over 12 months or less.
2025 Key Dates


*Based on EST time
2025 Grant Awardees
Cycle 1Cycle 2 Avencia Sanchez-Mejias Integra Therapeutics Gene and Cell Therapy
Avencia Sanchez-Mejias Integra Therapeutics Gene and Cell Therapy Bomyoung LeeFred Hutchinson Cancer Research Center (FHCRC) Gene and Cell Therapy
Bomyoung LeeFred Hutchinson Cancer Research Center (FHCRC) Gene and Cell Therapy Courtney Lane-Donovan University of California, San Francisco (UCSF) Diagnostics
Courtney Lane-Donovan University of California, San Francisco (UCSF) Diagnostics Edward Emmott University of Liverpool Diagnostics
Edward Emmott University of Liverpool Diagnostics Enping Qu ClearVision Therapeutics, Inc Gene and Cell Therapy
Enping Qu ClearVision Therapeutics, Inc Gene and Cell Therapy Jacob Brenner University of Pennsylvania Gene and Cell Therapy
Jacob Brenner University of Pennsylvania Gene and Cell Therapy Jamie Cate University of California, Berkeley Antibody Drug Discovery
Jamie Cate University of California, Berkeley Antibody Drug Discovery Julian Reyes Vera Biotechnologies AI Drug Discovery
Julian Reyes Vera Biotechnologies AI Drug Discovery Lennart NickelSwiss Federal Institute of Technology Lausanne (EPFL) AI Drug Discovery
Lennart NickelSwiss Federal Institute of Technology Lausanne (EPFL) AI Drug Discovery Leyuan Ma University of Pennsylvania (UPenn) Vaccine Development
Leyuan Ma University of Pennsylvania (UPenn) Vaccine Development Le CongStanford University Gene and Cell Therapy
Le CongStanford University Gene and Cell Therapy Maddie Williams Sift Biosciences Vaccine Development
Maddie Williams Sift Biosciences Vaccine Development Monica Lisa Fernandez-QuinteroScripps Research Antibody Drug Discovery
Monica Lisa Fernandez-QuinteroScripps Research Antibody Drug Discovery Nicole PekCincinnati Children's Hospital Medical Center (CCHMC) Gene and Cell Therapy
Nicole PekCincinnati Children's Hospital Medical Center (CCHMC) Gene and Cell Therapy Russell Vance UC Berkeley Vaccine Development
Russell Vance UC Berkeley Vaccine Development Suki Albers-Fomenko Universität Hamburg Small Nucleic Acid Drug
Suki Albers-Fomenko Universität Hamburg Small Nucleic Acid Drug Timothy Riley310 AI AI Drug Discovery
Timothy Riley310 AI AI Drug Discovery Yonatan Lipsitz Lilium Therapeutics Inc (Labcentral) Gene and Cell Therapy
Yonatan Lipsitz Lilium Therapeutics Inc (Labcentral) Gene and Cell Therapy2024 Grant Awardees
Cycle 1Cycle 2Cycle 3Cycle 4



 Eben Alsberg &
Eben Alsberg &
Sriramya Ayyagari University of Illinois at Chicago Gene and Cell Therapy More Details
 Giuseppe A. Sautto Cleveland Clinic Florida Research and Innovation Center Vaccine Development More Details
Giuseppe A. Sautto Cleveland Clinic Florida Research and Innovation Center Vaccine Development More Details





 Mark Sellmyer University of Pennsylvania Diagnostics
Mark Sellmyer University of Pennsylvania Diagnostics Nazia Samudh University of the Witwatersrand Vaccine Development
Nazia Samudh University of the Witwatersrand Vaccine Development Oleg GangColumbia University Other
Oleg GangColumbia University Other Peter Pushko Medigen, Inc. Vaccine Development
Peter Pushko Medigen, Inc. Vaccine Development Possu HuangStanford University Other
Possu HuangStanford University Other



 Beatrice Xuan Ho Agency for Science Technology and Research Gene and Cell Therapy
Beatrice Xuan Ho Agency for Science Technology and Research Gene and Cell Therapy
 Cobus Zwiegelaar Azargen Biotechnologies Plant Science
Cobus Zwiegelaar Azargen Biotechnologies Plant Science Giuseppe Pugliese UNIMORE, University of Modena and Reggio Emilia Gene and Cell Therapy
Giuseppe Pugliese UNIMORE, University of Modena and Reggio Emilia Gene and Cell Therapy Isabel Chu Zipcode Bio Other
Isabel Chu Zipcode Bio Other Jennifer Clinton Baylor College of Medicine Vaccine Development
Jennifer Clinton Baylor College of Medicine Vaccine Development Jiang-Hui Wang UMass Medical School (UMMS) Gene and Cell Therapy
Jiang-Hui Wang UMass Medical School (UMMS) Gene and Cell Therapy Lydia Lee University College London Cancer Institute Gene and Cell Therapy
Lydia Lee University College London Cancer Institute Gene and Cell Therapy Markus Depfenhart North- West University, South Africa Vaccine Development
Markus Depfenhart North- West University, South Africa Vaccine Development
 Matteo Dal Peraro University of the Witwatersrand Vaccine Development
Matteo Dal Peraro University of the Witwatersrand Vaccine Development
 Mihue Jang Korea Institute of Science and Technology Gene and Cell Therapy
Mihue Jang Korea Institute of Science and Technology Gene and Cell Therapy Muneaki Nakamura Ivy Natal Gene and Cell Therapy
Muneaki Nakamura Ivy Natal Gene and Cell Therapy
 Nigus Ambaye University of Maryland, Baltimore (UMB) Antibody Drug Discovery
Nigus Ambaye University of Maryland, Baltimore (UMB) Antibody Drug Discovery
 Pooja Kaur Ecole Polytechnique Fédérale de Lausanne (Switzerland) Antibody Drug Development
Pooja Kaur Ecole Polytechnique Fédérale de Lausanne (Switzerland) Antibody Drug Development Ramani Ramchandran Medical College of Wisconsin (MCW) Diagnostics
Ramani Ramchandran Medical College of Wisconsin (MCW) Diagnostics
 Rogelio Hernandez-Lopez Stanford University Gene and Cell Therapy
Rogelio Hernandez-Lopez Stanford University Gene and Cell Therapy Santanu Sasidharan UBC-Vancouver Antibody Drug Discovery
Santanu Sasidharan UBC-Vancouver Antibody Drug Discovery Shoubao Ma City of Hope National Medical Center Gene and Cell Therapy
Shoubao Ma City of Hope National Medical Center Gene and Cell Therapy Sreekumar Balan Icahn School of Medicine at Mount Sinai Antibody Drug Discovery
Sreekumar Balan Icahn School of Medicine at Mount Sinai Antibody Drug Discovery Victoria McGilligan Ulster University Antibody Drug Discovery
Victoria McGilligan Ulster University Antibody Drug Discovery Volker Thiel University of Bern, Institut für Virologie und Immunologie(IVI) Other
Volker Thiel University of Bern, Institut für Virologie und Immunologie(IVI) Other Wim Maes Katholieke Universiteit Leuven (KU Leuven) / PharmAbs Antibody Drug Discovery
Wim Maes Katholieke Universiteit Leuven (KU Leuven) / PharmAbs Antibody Drug Discovery Xiaoyan Ding Klinikum der Universität MünchenRegion Gene and Cell Therapy
Xiaoyan Ding Klinikum der Universität MünchenRegion Gene and Cell Therapy Adam Thomas Burnet Institute Vaccine Development
Adam Thomas Burnet Institute Vaccine Development Andreas Stengl LMU Munich Munich, German Antibody Drug Discovery
Andreas Stengl LMU Munich Munich, German Antibody Drug Discovery Behnam Rashidieh Mater Gene and Cell Therapy
Behnam Rashidieh Mater Gene and Cell Therapy Chao Ma Cleveland Clinic Gene and Cell Therapy
Chao Ma Cleveland Clinic Gene and Cell Therapy Cesare Cejas MFX Ltd Gene and Cell Therapy
Cesare Cejas MFX Ltd Gene and Cell Therapy Christopher Batho University of Cambridge Gene and Cell Therapy
Christopher Batho University of Cambridge Gene and Cell Therapy Christopher J. Vavricka Tokyo University of Agriculture and Technology Antibody Drug Discovery
Christopher J. Vavricka Tokyo University of Agriculture and Technology Antibody Drug Discovery Coralie Werbrouck The University of Sydney Gene and Cell Therapy
Coralie Werbrouck The University of Sydney Gene and Cell Therapy Emilie Stermann CNRS Antibody Drug Discovery
Emilie Stermann CNRS Antibody Drug Discovery Eric Shifrut Tel Aviv University Gene and Cell Therapy
Eric Shifrut Tel Aviv University Gene and Cell Therapy Ferenc Scheeren Leiden University Medical Center (LUMC) Antibody Drug Discovery
Ferenc Scheeren Leiden University Medical Center (LUMC) Antibody Drug Discovery Filipe Pinto i3S - Instituto de Investigação e Inovação da Universidade do Porto Others
Filipe Pinto i3S - Instituto de Investigação e Inovação da Universidade do Porto Others Jack Rudrum Massachusetts Institute of Technology Vaccine Development
Jack Rudrum Massachusetts Institute of Technology Vaccine Development Jagannath Erisyon Inc. Others
Jagannath Erisyon Inc. Others Jennifer Treweek University of Southern California Gene and Cell Therapy
Jennifer Treweek University of Southern California Gene and Cell Therapy Jeremiah Kim UChicago Vaccine Development
Jeremiah Kim UChicago Vaccine Development JoseGarrido Mesa Hospital 12 de Octubre Antibody Drug Discovery
JoseGarrido Mesa Hospital 12 de Octubre Antibody Drug Discovery Jose Martinez-Navio University of Miami - Miller School of Medicine Gene and Cell Therapy
Jose Martinez-Navio University of Miami - Miller School of Medicine Gene and Cell Therapy Joshua Krueger University of Minnesota Gene and Cell Therapy
Joshua Krueger University of Minnesota Gene and Cell Therapy Lay Khoon Too University of Sydney Gene and Cell Therapy
Lay Khoon Too University of Sydney Gene and Cell Therapy Le Cong Stanford University Vaccine Development
Le Cong Stanford University Vaccine Development Neeraj Saini MD Anderson Cancer Center Gene and Cell Therapy
Neeraj Saini MD Anderson Cancer Center Gene and Cell Therapy Nicole Paulk Siren Biotechnology, Inc. Gene and Cell Therapy
Nicole Paulk Siren Biotechnology, Inc. Gene and Cell Therapy Patrick Holec Fletcher Biosciences Antibody Drug Discovery
Patrick Holec Fletcher Biosciences Antibody Drug Discovery Pawel Stocki Ossianix Gene and Cell Therapy
Pawel Stocki Ossianix Gene and Cell Therapy Shantanu Kumar Revision Bio Gene and Cell Therapy
Shantanu Kumar Revision Bio Gene and Cell Therapy Sushama Telwatte University of Melbourne Gene and Cell Therapy
Sushama Telwatte University of Melbourne Gene and Cell Therapy Xiao Huang Drexel University Gene and Cell Therapy
Xiao Huang Drexel University Gene and Cell Therapy Xiaojing Gao Stanford Diagnostics
Xiaojing Gao Stanford Diagnostics Yoichi Furuya Pictor Ltd Diagnostics
Yoichi Furuya Pictor Ltd Diagnostics Bassel Akache National Research Council of Canada (NRC) Vaccine Development
Bassel Akache National Research Council of Canada (NRC) Vaccine Development Bryan Bryson MIT/Ragon Institute Vaccine Development
Bryan Bryson MIT/Ragon Institute Vaccine Development Chloe Christensen Children's Hospital Orange County Gene and Cell Therapy
Chloe Christensen Children's Hospital Orange County Gene and Cell Therapy Christopher Lawrence Moore GentiBio Gene and Cell Therapy
Christopher Lawrence Moore GentiBio Gene and Cell Therapy Diem TRANMedical Genetics Institute Vaccine Development
Diem TRANMedical Genetics Institute Vaccine Development Fabio MammanoUniversity of Padova, Dept. of Physics and Astronomy Antibody Drug Discovery
Fabio MammanoUniversity of Padova, Dept. of Physics and Astronomy Antibody Drug Discovery Ivan LoncarevicQUANTBIORES A/S (QBR) Vaccine Development
Ivan LoncarevicQUANTBIORES A/S (QBR) Vaccine Development Lajos MatesBiological Research Center, Szeged OthersLiang Feng Duke University Gene and Cell Therapy
Lajos MatesBiological Research Center, Szeged OthersLiang Feng Duke University Gene and Cell Therapy Lucy BarthoThe University of Melbourne Diagnostics
Lucy BarthoThe University of Melbourne Diagnostics Maider Zabala UgaldeOnena Medicines S.L. Antibody Drug Discovery
Maider Zabala UgaldeOnena Medicines S.L. Antibody Drug Discovery Miriam LongoFondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico Milano, Lombardia Gene and Cell Therapy
Miriam LongoFondazione IRCCS Cà Granda, Ospedale Maggiore Policlinico Milano, Lombardia Gene and Cell Therapy Naresha Saligrama Washington University in Saint Louis Others
Naresha Saligrama Washington University in Saint Louis Others Natividad Garrido MesaKingston University Others
Natividad Garrido MesaKingston University Others Ori Zelichov ZipBio Inc. Others
Ori Zelichov ZipBio Inc. Others Reza ShahbaziIndiana University School of Medicine Gene and Cell Therapy
Reza ShahbaziIndiana University School of Medicine Gene and Cell Therapy Ritchie Chen University of California, San Francisco (UCSF) Gene and Cell Therapy
Ritchie Chen University of California, San Francisco (UCSF) Gene and Cell Therapy Ryma ToumiSeattle Children's Research Institute Gene and Cell Therapy
Ryma ToumiSeattle Children's Research Institute Gene and Cell Therapy Sakthi Moorthy CCRM Gene and Cell Therapy
Sakthi Moorthy CCRM Gene and Cell Therapy Shimobi Onuoha Chimeris UK Ltd Gene and Cell Therapy
Shimobi Onuoha Chimeris UK Ltd Gene and Cell Therapy Silvia gomez-sebastian Universidad Autonoma de Madrid Gene and Cell Therapy
Silvia gomez-sebastian Universidad Autonoma de Madrid Gene and Cell Therapy Torsten MeissnerBIDMC Gene and Cell Therapy
Torsten MeissnerBIDMC Gene and Cell Therapy Tyler Renner Translational Biosciences Department, Human Health Therapeutics, National Research Council Others
Tyler Renner Translational Biosciences Department, Human Health Therapeutics, National Research Council Others Uri Manor UCSD Gene and Cell Therapy
Uri Manor UCSD Gene and Cell Therapy William MacDonaldRhoGen Inc. Antibody Drug Discovery
William MacDonaldRhoGen Inc. Antibody Drug Discovery Yijun Pan The University of Melbourne, Florey Department of Neuroscience and Mental Health Diagnostics
Yijun Pan The University of Melbourne, Florey Department of Neuroscience and Mental Health Diagnostics Yoshinori HasegawaKAZUSA DNA Research Institute Plant Science
Yoshinori HasegawaKAZUSA DNA Research Institute Plant Science Yue Clare Lou Phase0, Inc. DBA Sift Biosciences Vaccine Development
Yue Clare Lou Phase0, Inc. DBA Sift Biosciences Vaccine Development Zizhang ShengColumbia University Irving Medical Center Antibody Drug Discovery
Zizhang ShengColumbia University Irving Medical Center Antibody Drug Discovery

Path to Your GenScript Life Science Research Grant
What We Do
MOMM Diagnostics develops high-sensitivity rapid diagnostic tests. MOMMs proprietary technology opens previously laboratory-based test for in clinic- and self-testing. Our first test in development is preXclude - a rapid test for preeclampsia, offering unprecedented sensitivity at the point-of-care. Preeclampsia is a significant health problem accounting for a third of obstetric costs (over 2 Billion USD) in the US alone. It affects 2-8% of all pregnancies worldwide and can lead to severe short- and long-term complications for both mother and baby. preXclude delivers immediate information to doctors during a pregnancy check-up helping them optimize treatment and reduce the stress and anxiety for expectant mothers.
How GenScript LSRG Helps
The money granted in the MOMMscript project will enable MOMM Diagnostics to utilise GenScript’s expertise in recombinant antibody development and manufacturing to secure the supply of highly sensitive and specific affinity reagents.
What We Do
The proteins in the AAA+ superfamily of ATPases are often described as molecular machines because they form oligomeric structures that harness chemical energy derived from ATP hydrolysis to perform mechanical works on proteins or nucleic acids. These molecular machines are found in all kingdoms of living organisms, and they have evolved to perform different functions, thereby participating in diverse cell regulation processes. My research is focused on elucidating the structural basis for the functions of these molecular machines, by understanding how their oligomeric structures and biochemical properties are coupled together. Many of the AAA+ proteins in higher organisms have evolved from bacterial ancestors with a significantly expanded ATPase region and form supramolecular complexes in cells. Investigating these megadalton complexes poses challenges, demanding innovative approaches such as gene constructs tailored for effective protein purification.
How GenScript LSRG Helps
I am excited about leveraging support from the LSRG-funded gene synthesis service to significantly advance these investigations.
What We Do
Adeno-Associated Viruses (AAVs) are the leading tool in current gene therapy. However, immune responses to AAVs limit their efficacy, reduces patient eligibility, and constitute a major limitation of their therapeutic application.
In this project, we leverage generative AI-based protein design to explore novel AAV capsids able to evade pre-existing immunity. To test the efficacy of infection of our in-silico designs, and to further train the model, we combined directed evolution of chimeric capsids and de novo capsid design. We then tested these new libraries using high-throughput methods in multiple cellular models, such as HEK293T and HepG2, both in presence and in absence of patient-derived sera. Testing with patient-derived sera reports on the ability of novel AAV capsids to evade pre-existing neutralizing antibodies that would decrease therapeutic efficacy.
By iterating generative modeling and screening with sera from a diverse population, we anticipate the discovery of capsid sequences with high probability of correct folding, high immune-evasion potential and a wide applicability across multiple ethnic groups.
How GenScript LSRG Helps
“Thanks to the LSRG grant, we could expand our repertoire of de novo synthesis to 100 synthetic sequences, and have them all synthesized and shipped to us. An endeavor otherwise not possible for an academic lab.”
“The LSRG grant enabled us to pursue two parallel strategies and effectively de-risk our original approach”
What We Do
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness, projected to affect 288 million people worldwide by 2040 due to the growing ageing population. Neovascular AMD (nAMD), the most severe form, leads to sudden central vision loss, caused by leaky blood vessels that bleed into the retina, causing tissue damage and scarring. The rapid onset and demographic of AMD result in high social and healthcare costs.
Although anti-VEGF therapies have revolutionized nAMD treatment, they involve frequent eye injections every 6-8 weeks that is burdensome and unpleasant for patients and caregivers, significantly impacting their quality of life. Importantly, a significant proportion of eyes with nAMD develop retinal/macular scarring and these eyes respond very poorly to anti-VEGF therapies with respect to preservation of vision.
Ikarovec is developing IKC203V, an AAV-based ocular gene therapy for nAMD. IKC203V encodes two secreted proteins: an Fc-fusion protein to neutralise VEGF, a key driver of neovascularisation and a single-chain variable fragment (ScFv) to neutralise CTGF, a key driver of fibrosis/scarring
The efficacy of the anti-CTGF component will be assessed in vivo using an optimised 2-stage laser CNV subretinal fibrosis model, and screened against other anti-fibrotics.
How GenScript LSRG Helps
We are thrilled to be working with GenScript to advance the development of our gene therapy. We have a long-standing relationship with GenScript in relation to plasmid cloning, and we are excited to try their vector manufacture services. This grant has allowed Ikarovec to expand our in vivo anti-fibrotic evaluation, which in turn will help complete a full data package suitable for clinical development.
What We Do
The main focus of our lab is on antibody discovery and characterization, especially for infectious diseases-associated pathogens, including hypervariable viruses such as influenza virus, SARS-CoV-2 and hepatitis C virus (HCV).
More in detail, the lab is equipped with >15 years of research experience focused on the selection, cloning, characterization and engineering of monoclonal antibodies (mAbs), of human or animal origin, and directed against infectious agents. In particular, the potential of these mAbs is investigated as therapeutic molecules, able to directly neutralize infectious pathogens, or as indirect molecules to be exploited for the development of prophylactic approaches and for the modeling of the humoral immune response. Moreover, their possible engineering in order to improve (i.e., their binding or stability characteristics) or gain new functions (such as in the case of chimeric antigen receptors, CARs), represents a promising and alternative approach for difficult-to-eradicate infectious pathogens. In this context, we are committed also in the field of innovative prophylactic countermeasures for the purpose of improving or developing new vaccines for hypervariable pathogens. In particular, we are investigating the mechanisms of protection elicited by standard of care and next-generation influenza vaccines and adopting different strategies for the development of a vaccine against HCV.
How GenScript LSRG Helps
The LSRG-funded GenScript services will benefit the research of our lab for generating the necessary molecular tools for the assessment of the immune response elicited by our experimental HCV vaccines.
What We Do
The liver’s role in drug metabolism makes it an ideal candidate to use for in vitro drug screening applications. Human liver organoids (HLO) are three-dimensional tissue constructs that recapitulate native cell architecture and functionality and have immense potential for screening new pharmaceutical therapies. However, HLO generation heavily relies on Matrigel, an ill-defined tumor-derived extracellular matrix (ECM) that contributes to varied HLO formation and function. Due to these limitations, evaluating the toxicity of new pharmaceutics can be challenging. For this project, we plan to generate a defined synthetic matrix with controlled presentation of ECM biosignals and mechanical signals that can be used to reliably form HLO. Tailored alginate matrices will be used as the biomaterial for developing a defined ECM, as cells cannot adhere to native alginate, and its mechanical properties can be independently controlled by introducing additional functional groups. We plan to use tailored alginate matrices, whose ECM biomechanics can be independently controlled from the matrix biosignals, to determine what signals are inductive for liver tissue formation. This work will be a critical step in increasing our understanding of signals involved in the formation of the liver and provide a new biomaterial design platform for consistently generating organoids.
How GenScript LSRG Helps
The GenScript services will allow us to systematically evaluate what role matrix biosignals and mechanics have on liver organoid formation in our biomaterial system. This information may then be used to reliably generate liver tissue constructs in vitro.
What We Do
Living cells are intricate information processors. Unlike electronic computers that rely solely on electrical signals to process information, living cells use a complex network of interacting biochemical and biophysical signals to drive biological function and achieve biological computation. These signals include ion concentrations, molecular messenger levels, protein activities, and gene regulations. They form signal transduction networks in cells and collectively convert cellular inputs into cellular outcomes by interacting in complex ways. Subtle defects in these processes are associated with a wide range of diseases. We (UMich Spatial Biodynamics: spatialbiodynamics.org) are developing new molecular tools and imaging technologies to measure and analyze these cellular signals, to ultimately provide mechanistic insights into mammalian cellular processes in healthy and diseased states.
How GenScript LSRG Helps
The LSRG-funded gene synthesis and cloning service at GenScript will help us to generate new molecular constructs and accelerate our efforts to design and validate novel molecular tools.
What We Do
TargetGene, founded in 2012 in Israel, is a pioneer in the gene-editing field, propelled by its proprietary best-in-class platform known as T-GEE. T-GEE enables SAFE modification of the patient’s own DNA. With T-GEE, genes can be added, deleted, or precisely replaced—effectively rewiring the transcriptome. We thus intend to correct genetic abnormalities or enhance specific cell types, ultimately leading to cures rather than chronic treatments.
Leveraging the advantages of T-GEE, we’re driving innovations in the development of safe and effective cell therapies for cancer, cardiovascular and immune-related diseases. Our commitment extends to achieving both pre-clinical and clinical readiness.
Our novel and proprietary T-GEE programmable molecular “DNA scissors” utilizes two guides and an obligatory dimeric nuclease to achieve its exquisite specificity. T-GEE solves the biggest problem of gene editing - off-target cleavage of unintended genes- and is predicted to be orders of magnitude more precise than CRISPR-Cas9, potentially creating a radically safer therapeutic tool.
TargetGene has an exceptionally rich and broad patent portfolio including 15 granted patents and multiple patents pending. We are strategically partnering in different cell therapies and are also key partners in CAR-T REX, a major EU-funded consortium focused on CAR-T therapies for solid tumors.
How GenScript LSRG Helps
With the assistance of the LSR grant we hope to get preliminary data on novel methods to further enhance the T-GEE platform and to subsequently broaden our patent portfolio.
What We Do
T lymphocytes are powerful immune cells capable of combating severe infections and, under certain conditions, attacking healthy organs. Over the past decades, significant advancements have enabled us to effectively redirect these cells against cancer. This approach has achieved tremendous success, particularly in hematological cancers and, more recently, in the treatment of rheumatological diseases. However, the complexity of solid tumors presents a unique challenge, necessitating more precisely defined genetic programs and sophisticated genome engineering techniques. Our research leverages cutting-edge genome engineering, including large-scale CRISPR screening, to decode complex genetic networks within T cells. By systematically altering genes and observing the resultant changes in T cell behavior, we aim to unravel the intricate genetic interactions that govern T cell function and effectiveness. This knowledge will be instrumental in designing the next generation of cancer therapies, tailored to overcome the specific challenges posed by solid tumors. Additionally, we are investigating novel gene targets and regulatory elements that could enhance T cell persistence and anti-tumor activity. Our ultimate goal is to develop innovative therapeutic strategies that harness the full potential of T cells, thereby improving outcomes for patients with various cancer types and advancing the field of immunotherapy.
How GenScript LSRG Helps
The GenScript Life Sciences Grant will help us shortening our iteration cycles as we design and test genomic T cell alterations in the fight against cancer.
What We Do
Inherited bone marrow diseases impact 1 in 500 Canadians. Hematopoietic stem cell transplantation (HCT) can be curative, but is limited by factors such as socioeconomic barriers, appropriate donor availability, toxicities, and risks of graft-vs-host disease. While ex-vivo gene editing technologies of hematopoietic stem and progenitor cells (HSPCs) are promising they can be expensive, time consuming and require specialized manufacturing facilities. In-vivo gene therapy could overcome these limitations by circumventing the need for HCT. However, targeted delivery into HSPCs is a challenge. The focus of our research proposal is to develop protocols for direct in-vivo gene editing of HSPCs for treatment of monogenic illnesses.
How GenScript LSRG Helps
Funding from LSRG-funded GenScript grant will cover costs associated with tools (gene editing machinery) required for gene editing of HSPCs.
What We Do
ProtOS is creating a generative AI based mRNA therapeutic development platform that designs coding mRNA sequences at the nucleotide level. These mRNA drugs code for expressible and functional proteins that discover and modulate disease pathways. Current proof-of-concept is focused on known cancer pathways, such as the MDM2-p53 interaction but scope may become wider in the medium term.
Target discovery and lead development are done by in-silico design of binders to specific epitopes of proteins involved in disease. The development process involves iterative LNP-mRNA/plasmid-DNA design cycles, utilizing high content imaging of cellular phenotypes to guide the AI models. Designs serve to both probe pathways and modulate them - functioning as a unified platform for target discovery and lead development. Key innovations are propietary nucleotide level mRNA generative models, and a reinforcement learning design loop guided by cellular imaging.
How GenScript LSRG Helps
The BLI assays performed at GenScript allow us to show that our in-silico protein design process is able to design binders at very high success probability. We can now use the results of this assay to guide our designs in the following cellular assays.
What We Do
Adeno-Associated Viruses (AAVs) are highly promising vectors for gene therapies due to their high efficiency, sustained expression, and low toxicity. However, robust delivery vehicles to the central nervous system (CNS) are lacking. My project aims to discover novel delivery mechanisms targeting diverse cell types within the CNS and explore how they respond to different transgenes. Over the past 25 years, the Schaffer lab has developed large AAV libraries through capsid diversification including error prone PCR, capsid shuffling, “loop swap”, and peptide display. Through directed evolution, I screened these libraries with clinically relevant human brain slices and non-human primates to identify CNS-specific AAV variants. Here, I propose to use next-generation sequencing (NGS) coupled with machine learning approaches to predict cell-specific, high infectivity AAV libraries. In summary, I aim to discover clinically relevant gene delivery approaches for various cell types within the CNS, ultimately treating complex neurological diseases, which currently have very limited therapies.
How GenScript LSRG Helps
GenScript award provides us a great opportunity to construct our AAV libraries and to explore the power of high-throughput DNA synthesis.
The flexibility of GenScript team and the fast turn-around time greatly benefit our project in moving forward with AAV screening and validating.
What We Do
Dr Kevin Sek is a cancer researcher and immunologist at the Peter MacCallum Cancer Centre under the supervision of Professor Phillip K. Darcy. Dr Sek’s research interest includes novel cell and gene-therapies for the treatment of cancer with a specific focus on adoptive CAR-T cell therapy for solid tumors. Utilizing gene and epigenome editing tools, as well as mRNA and Lipid Nanoparticle delivery platforms, he seeks to target fundamental T cell biology and the tumor microenvironment to enhance CAR-T cell therapies for various malignancies. He places a strong emphasis on translational research, with several pre-clinical adoptive therapies and drug pipelines in development for biotech companies Prescient and Myeloid Therapeutics.
How GenScript LSRG Helps
This LSRG grant provides me with the funding to kick-start a high-risk, high-reward research project that is at the forefront of cancer immunotherapy. GenScript’s high quality and rapid plasmid, mRNA and AAV services has drastically reduced the time it has taken to translate ideas into real-world feasibility data which will attract further funding and development.
What We Do
African swine fever (ASF) is a deadly transboundary disease that spreads through pigs, boars, and various fomites. When ASF is detected on a farm, the entire pig herd must be destroyed, and this has a devastating impact on the global pork industry. To date, there are three ASF vaccines at different stages of commercialization. Vaccines add incredible value to the industry and biosecurity programs worldwide, but there is a critical need to differentiate infected from vaccinated animals (DIVA), also known as a DIVA test. There are no commercial ASF DIVA tests, and therefore, current ASF diagnostics could produce a false positive result. Herein, we propose to develop technology for enzyme linked immunosorbent assays (ELISA) ASF DIVA tests against ASF-delta-MGF and ASF-delta-9GL/UK vaccines. Our project will employ GenScript’s peptide synthesis and protein production services to generate vaccine-specific antigens for ELISA development. We will collaborate with ASF research and diagnostics laboratories in our network to evaluate each ELISA. Our project is an important step towards providing the pork industry with ASF DIVA tests, which will impact the ability to manage disease and reduce the economic impacts of ASF.
How GenScript LSRG Helps
The LSRG-funded GenScript services will significantly reduce the cost of ELISA development for this project. By outsourcing peptide synthesis and protein production, we are saving valuable time and effort to focus on R&D.
What We Do
Cellular immunotherapy, particularly with T cells engineered to express chimeric antigen receptors (CAR-T cells), has shown significant promise in treating certain hematological malignancies. However, the need for effective therapies against other blood cancers and solid tumors remains critical. Our research focuses on developing a novel approach to cancer immunotherapy by genetically enhancing "adaptive" Natural Killer (NK) cells using CRISPR/Cas9 technology. Adaptive NK cells are a unique subset of NK cells found in a significant proportion of cytomegalovirus (CMV) positive individuals. These cells possess robust cytotoxic potential and a distinct receptor repertoire that enhances their ability to target and kill cancer cells. Our project aims to create an off-the-shelf NK cell therapy by editing three key aspects of these cells: enhancing cytotoxic potency, expanding the range of targetable cancer types, and extending cell persistence. By leveraging CRISPR/Cas9-mediated gene knock-outs and knock-ins, we intend to boost the natural properties of ADAPT-NK cells, ultimately advancing a powerful and versatile cell therapy platform for treating both hematological malignancies and solid tumors.
How GenScript LSRG Helps
The LSRG-funded GenScript services will significantly enhance our project by providing high-precision CRISPR/Cas9 gene editing tools and reagents, enabling us to execute multiple, targeted genetic modifications in adaptive NK cells with greater accuracy and efficiency. This will be crucial for developing a potent and scalable NK cell therapy for cancer treatment.
What We Do
Alzheimer's disease (AD) is the most common form of dementia and progressively affects memory, thinking and daily activities. AD has been long characterized with two hallmarks in the brain: neurofibrillary tangles in neurons and extracellular amyloid plaques, which are composed of hyperphosphorylated tau and amyloid beta respectively. There is mounting evidence for adaptive immune responses in people who have AD. However, the role of adaptive immunity in AD remains largely unknown. Our large cohort and multi ancestry GWAS have identified a protective effect of human leukocyte antigen (HLA)-DRB1*04 sub alleles, strongest with DRB1*04:04 and DRB1*04:07, intermediary with DRB1*04:01 and DRB1*04:03, and absent for DRB1*04:05. We found a particular piece of peptide from tau bound to DRB1*04:04 and DRB1*04:01 but not DRB1*04:05 only when it was modified by acetylation at lysine (K) 311, namely PHF6 aK311. We hypothesize that there are specific T cell immune responses to PHF6 aK311 presented by DRB1*04:04 . Our project is to characterize the role of these T cells in AD.
How GenScript LSRG Helps
The GenScript LSRG will help us synthesize overlapping peptides from tau and other antigens with high purity, including post-translational modifications (PTMs), and facilitate the isolation and characterization of antigen-specific T cells using multimer HLAs.
What We Do
Chimeric antigen receptor (CAR) T cells, designed with specific costimulatory and signaling domains to target tumor-specific antigens (TSAs) with single-chain variable fragment (scFv) have revolutionized the treatment of hematologic malignancies. However, their efficacy against solid tumors is limited by three major obstacles: (1) desmoplasia, a fibrotic barrier that restricts CAR T cell infiltration; (2) an immunosuppressive tumor microenvironment (TME) that suppresses CAR T cell activity; and (3) the shedding of TSAs, such as N-terminal mesothelin (MSLN), which generates decoy molecules that impair CAR T cell function. Transforming growth factor-β (TGF-β) signaling via TGFBR2 exacerbates desmoplasia and inhibits CAR T cell function, while the Vdomain Ig suppressor of T cell activation (VISTA) immune checkpoint pathway further suppresses immune responses, including those of CAR-effector cells. Our research focuses on developing neural network-optimized costimulatory domains to enhance tumor-specific cytotoxicity, incorporating exhaustion-resistant signaling mutations, and cloning a shed-MSLN-resistant scFv. Additionally, we aim to counteract TGF-β signaling through CRISPR/Cas9-mediated TGFBR2 knockout and target the VISTA pathway using RNA interference (RNAi). We believe these strategies will significantly enhance the effectiveness of CAR therapy against MSLN-expressing solid tumors, such as ovarian cancer.
How GenScript LSRG Helps
Thanks to the LSRG-funded GenScript services and reagents, we will be able to quickly develop and validate the proposed advanced CAR T cell platform. This support will also facilitate the use of cutting-edge tools, such as RNAi technology, machine learning designed CAR domains and tumor spheroid models, to accelerate preclinical testing and bring innovative therapies for ovarian cancer closer to clinical application.
What We Do
My research focuses on understanding the role of T cells in the immune response to control Mycobacterium tuberculosis infection. I am particularly interested in how T cells contribute to controlling infection, with the ultimate goal of using these insights to inform the design of novel TB vaccines. To this end, I have been investigating whether individuals who control M. tuberculosis infection possess specific T cell clonotypes in higher abundance compared to those who progress to disease. Using single-cell TCR sequencing, I have identified a donor-unrestricted T cell clonotype that appears to be enriched in individuals who effectively control infection.
How GenScript LSRG Helps
Support from the LSRG from GenScript has enabled me to develop a custom monoclonal antibody to comprehensively characterize this donor unrestricted T cell. This funding is enabling the development of a critical research tool that I hope will generate key preliminary data to support further studies and unlock additional opportunities for larger-scale funding to advance this work.
What We Do
We are developing minimally invasive, site-specific labeling technologies for specific post-translationally modified forms of proteins and cellular translation events using non-canonical amino acids (ncAA) incorporated via the genetic code expansion (GCE) technology. One roadblock to wider use of GCE for protein and proteoform labeling is the need to screen multiple incorporation sites of the amino acid to identify optimal label sites for each species of interest. Such an exercise is necessary not only to maximize protein yields but also to ensure proper protein structure, stability, and function after ncAA incorporation, but is known to be unpredictable and tedious. Previous computational tools to predict ncAA incorporation sites relied on analysis of RNA sequences which permit translation readthrough, but have yet to be integrated with effects the ncAA may have to the overall protein structure and stability.
How GenScript LSRG Helps
LSRG from Genscript helps us massively expand the library size of testable incorporation sites for non-canonical amino acids, and will enable us to develop better predictive tools for optimal incorporation sites of ncAAs into proteins and proteoforms.
What We Do
KiraGen Bio is transforming cancer treatment with an AI-powered platform for designing multiplex gene-edited CAR-T cell therapies that overcome the immunosuppressive barriers of solid tumor microenvironments (TME). Focused on glioblastoma multiforme (GBM)—an aggressive brain cancer with a median survival of just 9-12 months—KiraGen addresses a critical unmet need in oncology. Its proprietary machine learning platform, KiraLOGIC, identifies optimal combinations of “TME Guards,” enabling CAR-T cells to evade suppressive pathways and restore robust anti-tumor activity. This innovative strategy redefines the potential of CAR-T therapies for GBM, laying the foundation for applications across other solid tumors. By integrating cutting-edge computational biology with next-generation gene editing, KiraGen is paving the way for transformative advancements in cancer care.
How GenScript LSRG Helps
The LSRG grant has been instrumental in advancing KiraGen Bio’s discovery platform for multiplex gene editing in CAR-T cell therapies. It enabled us to generate and test novel CAR constructs while expanding our toolbox with essential gene-editing materials. These resources have been critical for exploring combinatorial biology strategies, feeding our AI-powered machine learning algorithm, and accelerating the development of KiraGen's multiplex-edited CAR-T cells tailored for glioblastoma multiforme (GBM).
What We Do
PartitionBio has developed a novel modality for the intracellular delivery of therapeutic molecules, especially of biologics such as nucleic acids (e.g. mRNA), proteins (e.g. antibodies) and macromolecular complexes (e.g. gene editing assemblies). Our non-viral approach is based on peptidomimetic oligomers of a design that allows them to undergo liquid-liquid-phase-separation and self-organise into droplet-like biomolecular condensates. These micron-sized nanostructures are capable of enriching macromolecular cargos, and of delivering their payload across the membranes of target cells, where they become available to carry out intracellular functions.
We believe that our platform technology can overcome the shortcomings of alternative drug delivery technologies and will enable the development of medicines for currently undruggable targets. We hope to accelerate the implementation of next-generation therapeutic modalities, such as nucleic acid-based vaccination, the use of intracellular antibodies, and gene therapy. Our approach affords various potential benefits, particularly a wide range of possible cargo types, high payloads, superior biocompatibility, and inherently low-cost manufacturing, formulation & storage.
How GenScript LSRG Helps
The LSRG funded services provides us with an unique opportunity to generate a library of highly pure molecular constructs, facilitating our efforts to validate our design process.
What We Do
Many human viruses encode proteases within their genomes that are essential for processing viral proteins into functional subunits, making them ideal targets for therapeutic intervention. In addition, these viral proteases can target human proteins for cleavage, often disabling the cell’s ability to respond to infection. In our lab we aim to determine the cleavage specificity of viral proteases, discover novel host targets of viral proteases, and understand how host protein cleavage impacts the immune response and viral replication. We also utilize evolutionary approaches to determine the host specificity of viral cleavage, including identifying instances in which human polymorphism affects whether a host protein is susceptible or resistant to cleavage. Interestingly, while several antagonized proteins lose their function upon cleavage, we have also identified cases in which proteins are “activated” by viral protease cleavage. We are thus focused on revealing the diverse mechanisms by which viral protease cleavage leads to host protein activation and the consequences of these events on species-specific antiviral immunity. Together, our work aims to further our knowledge of viral proteases as therapeutic targets and understand the consequences of protease-host interactions in terms of host susceptibility to circulating and emerging (e.g. zoonotic) viruses.
How GenScript LSRG Helps
Funding from GenScript is critical to our goal of increasing the diversity of virally-encoded proteases we can test for activity, and the diversity of host proteins that we can test for cleavage susceptibility and function. Using their gene synthesis services, we will greatly expand the number of protease-host interactions we can identify, and the depth with which we can understand how host and viral evolution shape these interactions.
What We Do
Plant immunity is the crucial process of how plants protect themselves against the pathogen attack. In our laboratory, we are focused on pattern-triggered immunity (PTI). During PTI, receptors on the plasma membrane recognize elicitors called pathogen (or damage)-associated molecular patterns (PAMPs; DAMPs). The PAMP/DAMP recognition by pattern-recognizing receptors (PRRs) triggers distinct immune responses, among which the Ca2+ influx and burst of reactive oxygen species (ROS) are typical fast responses. In collaboration with the Laboratory of Plant Cell Morphogenesis at Biology Centre CAS in České Budějovice, we are investigating the role of Ca2+ in ROS burst. For that purpose, we use a unique collection of Arabidopsis thaliana mutants. Additionally, we are focused on investigating immune responses (especially PTI) in breadseed poppy (Papaver somniferum), a traditional crop in the Czech Republic. We believe detailed knowledge about poppy immunity would help increase its resistance to pathogens on the field.
How GenScript LSRG Helps
Thanks to the LSRG's support, we can screen a wide range of peptide elicitors in triggering ROS burst in Arabidopsis and poppy. In addition, we can investigate the influence of Ca2+ on ROS bursts using the peptides provided by GenScript.

-



































