-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Products
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Online Shop
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
News & Blogs » Peptide News » Challenges in Neoantigen Peptide Manufacturing | GenScript
Navigating the Challenges and Regulatory Landscape of Neoantigen Peptide Cancer Vaccines

Author: Gordon Li
Director of Peptide and R&D Department
A neoantigen peptide drug is a cutting-edge cancer immunotherapy designed to activate the patient’s immune system. By helping target tumor-specific antigens, these peptides stimulate a robust anti-cancer immune response.
When dosed in patients, a neoantigen peptide is presented to the immune cells, such as T-cells. Because neoantigen peptides are, by definition, new and foreign antigens, the immune cells recognize them.
Once stimulated, T-cells mount a response against the cancer cells expressing these neoantigens. This can lead to the killing of cancer cells and potentially the control or elimination of the tumor. 1,2
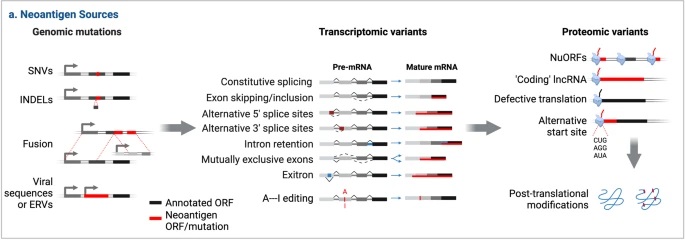
How are neoantigens produced. Neoantigens are novel proteins formed in tumor cells through various mechanisms. These may be genomic events, such as single-nucleotide variations (SNVs), base insertion/deletions (In/Dels), and gene fusions. Additionally, neoantigens may arise during transcription events, such as through alternative splicing of the pre-mRNA. Lastly, neoantigens may also form at the protein level due to dysregulated translational and post-translational events.1,3Retrieved from Figure 2, only panel a is shown (Xie et al. 2023). http://creativecommons.org/licenses/by/4.0/
A neoantigen peptide drug can be universal or public. This happens when the tumor antigen is present in the cancer cells of many patients. Public neoantigens come from driver mutations in key areas of the genome or hot spots. A definite advantage of public neoantigens is that they can be used in vaccine development for more patients.
In contrast, individualized neoantigen peptide drugs target private tumor antigens and are highly personalized. Creating these individualized therapies poses much more of a challenge because they are designed specifically for each patient.
Conventionally, developing personalized neoantigen peptide cancer vaccines may require the manufacturing of more than 20 different peptides for a single patient. Moreover, they must be custom-manufactured on a smaller scale. Lastly, the timeline for neoantigen peptide manufacturing is expedited to ensure their prompt delivery to patients in dire need.5

Expedited timeline for delivery of neoantigen peptide vaccines to patients. The workflow for the manufacturing of individualized neoantigen peptide vaccines is exemplified by the production of FRAME-001 for patients with advanced non-small cell lung cancer. This personalized neoantigen peptide vaccine required the synthesis of 24 patient-specific neoantigen peptides within a short timeframe of 19 days. The timeline for the complete vaccine manufacturing process was 48 days. Figure 1 retrieved without modifications from Oosting et al., 2022.6https://creativecommons.org/licenses/by/4.0/
Before we dive into the challenges in neoantigen peptide synthesis, we should understand the main regulatory concerns of personalized peptide drugs.
Ensuring Quality and Safety in Neoantigen Peptide Manufacturing
Advances in neoantigen-based cancer vaccines have pushed the expedited approval of these immunotherapies. The FDA in the US and the EMA in Europe oversee the regulation of clinical studies. They also regulate the approval of neoantigen cancer vaccines.2
The goal of the currently established regulations is to safeguard patients receiving these highly personalized cancer therapies. Stringent regulations ensure the safety, quality, and efficacy of neoantigen peptide cancer vaccines.
1. Safety and Toxicity
l Autoimmunity Risk: A major concern with neoantigen peptides is their potential to trigger autoimmune reactions. Researchers design these peptides to activate the immune system against tumor-specific antigens. However, some neoantigens may share structural or sequence similarities with self-antigens in normal tissues.
Therefore, there is still a risk of cross-reactivity. This means activated T-cells might mistakenly attack similar antigens in healthy tissues.
l Immune-Related Adverse Events (irAEs): As immunotherapeutic agents, neoantigen peptides can trigger immune-related adverse events, including inflammation in various organs such as the lungs, liver, and intestines. Robust regulatory frameworks are essential to ensure that healthcare providers are well-equipped to monitor, manage, and mitigate these potentially serious side effects effectively. Regulatory agencies will closely assess whether proper guidelines and protocols are in place to safeguard patient safety.
2. Consistency and Purity
l Consistent Peptide: The manufacturing process of neoantigen peptides must be able to produce a consistent product. Any variation in the peptide sequence, conformation, or purity could affect its efficacy and safety.
l High-Purity: Manufacturers must ensure high peptide purity levels. This requirement minimizes the presence of impurities. Components such as truncated peptides, side-reaction products, or contaminants could have adverse effects on patients.
Challenges in Standardizing Individualized Neoantigen Peptide Production
Drug regulatory agencies require manufacturing suppliers of individualized neoantigen peptides to adhere to good manufacturing practice (GMP)standards, and implement robust quality control measures.
1. Manufacturing and Quality Assurance
Customization and Standardization: Balancing the need for customization for each patient while maintaining some level of standardization in the manufacturing process is challenging. Regulators must ensure that despite the individualized nature of the peptides being manufactured, there are consistent quality control measures in place.
l Supply Chain and Traceability: With individualized treatments, ensuring a reliable supply chain and proper traceability of the neoantigen peptide from raw materials to the final product administered to the patient is extremely necessary. This is crucial for quality control and, in case of any adverse events, being able to trace back the potential source of problems.
2. Qualified testing method of purity (related substances)
High diversity: Neoantigen peptides are highly individualized. Each patient may have a unique set of neoantigen peptides based on their specific tumor mutations. This means that there is a vast variety of peptide sequences, making it difficult to develop a one-size-fits-all testing method.
Different peptide sequences can have unique physical and chemical properties. These properties can affect how well manufacturers resolve, and detect some peptides during purity analysis.
Instability: Neoantigen peptides may be relatively unstable and prone to degradation or modification. This instability can further complicate accurate peptide purity testing.
Evolving Regulatory Landscape of Neoantigen Peptide Production
Another current issue on individualized neoantigen peptide synthesis is regulatory and standardization issues across regions.
Lack of established standards
There are currently no well-established international standards for the purity testing of individualized neoantigen peptide drugs. Without clear reference materials and standard operating procedures, it is challenging to develop and validate accurate and reliable testing methods. Each laboratory may use different methods or parameters, leading to inconsistent results.
Regulatory harmonization requirements
Regulatory agencies are still in the process of formulating appropriate guidelines for the evaluation of these novel drugs. The lack of clear regulatory requirements regarding peptide purity testing makes it difficult for pharmaceutical companies to develop testing methods that can meet future regulatory expectations.
Conclusion
In summary, compliance with regulatory guidance is paramount to ensuring the safety and efficacy of neoantigen peptide drugs. Regulatory agencies demand consistent quality, which is crucial for maintaining the integrity and reliability of treatments. Although achieving this requirement perfectly is challenging, especially for individualized neoantigen peptide drugs manufactured and tested under the pressure of urgent needs for advanced-stage cancer patients, adherence to these guidelines is essential to safeguard patient health and optimize therapeutic outcomes.
Looking for reliable solutions in neoantigen peptide manufacturing?
Contact our peptide team to learn about GenScript neoantigen solutionsRelevant Resources

Reference
-
Xie, N., et al. (2023). Neoantigens: promising targets for cancer therapy. Sig Transduct Target Ther . https://doi.org/10.1038/s41392-022-01270-x
-
Li, X., et al. (2023). Neoantigen cancer vaccines: a new star on the horizon. Cancer Biol Med. 2023 Dec 29;21(4):274–311. https://doi.org/10.20892/j.issn.2095-3941.2023.0395
-
Bräunlein E, Krackhardt AM. (2017). Identification and Characterization of Neoantigens As Well As Respective Immune Responses in Cancer Patients. Front Immunol. https://doi.org/10.3389/fimmu.2017.01702
-
Hao, Q., et al. (2024). Development and Clinical Applications of Therapeutic Cancer Vaccines with Individualized and Shared Neoantigens. Vaccines. https://doi.org/10.3390/vaccines12070717
-
Zhang, T., Kurban, E., & Wang, Z. (2023). Neoantigens: The Novel Precision Cancer Immunotherapy. Biologics. https://doi.org/10.3390/biologics3040017
-
Oosting, L. T., et al. (2022). Development of a Personalized Tumor Neoantigen Based Vaccine Formulation (FRAME-001) for Use in a Phase II Trial for the Treatment of Advanced Non-Small Cell Lung Cancer. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14071515
Subscribe to Receive Updates
& Promotions From GenScript* We'll never share your email address with a third-party.
Latest News & Blogs
Find More Peptide NewsUnveiling the Potential of GLP-1 Receptor Agonists and Multi-Target Therapies

Challenges in Neoantigen Peptide Manufacturing | GenScript

Challenges & Innovations in Neoantigen Peptide Vaccine Production

The Clinical Application of Neoantigens: A Promising Frontier in Cancer Immunotherapy

Delving into the World of Cyclic Peptides: Harnessing the Power of Circular Molecules for Innovative Therapeutic Solutions

Ensure Safe & Effective Peptide Drugs: Mastering GMP Compliance for Quality Control


-



































