-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Products
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Online Shop
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
News & Blogs » CRISPR News » Theory to action: Applying CRISPR in Research
Theory to action: Applying CRISPR in Research
Traditional Gene Knockout
As described above, the CRISPR-Cas complex generates a targeted double-strand break, creating an opportunity for gene editing.
Gene knockout (KO) experiments exploit flaws in the cell’s native repair mechanism, non-homologous end joining (NHEJ). Imperfectly repaired genes with insertions or deletions of base pairs will result in frameshift mutations, rendering the gene and its corresponding protein non-functional. KO guides are algorithmically designed and ranked for both specificity (low homology with any other genomic sequence) and functionality (targeting a portion of the gene most likely to result in functional protein KO). CRISPR KO can be applied in many research scenarios, from basic screening, functional studies of a gene, bioengineering, agricultural biotechnology, to developing therapeutic gene and cell therapeutic drugs.
Increasing CRISPR/Cas9 Editing Specificity
CRISPR Cas9 based gene knockout relies on the specificity of both the sgRNA and the Cas9 protein. Poorly designed sgRNA and traditional wildtype Cas9 would lead to editing at undesired genome locations, which are considered as off-target edits. Many Cas9 variants have been developed for enhanced specificity. For example, the eSpCas9(1.1), also referred to as SpCas9 (K848A/K1003A/R1060A), structurally engineered from Feng Zhang lab contains alanine mutations that weaken the bounding between the HNH/RuvC groove and the none targeting DNA strand, reducing off-target effects by over 10-fold while maintaining robust on-target genome editing efficiency (Slaymaker et al. 2016).
Researchers have also engineered Cas9 variants to “nick” a single DNA strand instead of creating double stranded DNA breaks. Two individual nickases targeting opposite DNA strands are necessary to generate a break site for gene editing, which significantly increases targeting specificity. For example, SpCas9 nickase (Cas9n D10A) contains a mutation allowing the endonuclease to create single-strand nicks, as opposed to DSBs. Pairing two opposite facing gRNA sequences with SpCas9 nickase can be an efficient method of gene editing that prevents unwanted indels from forming.

Novel Editing Technologies Without DSBs (Prime Editing/Base Editing)
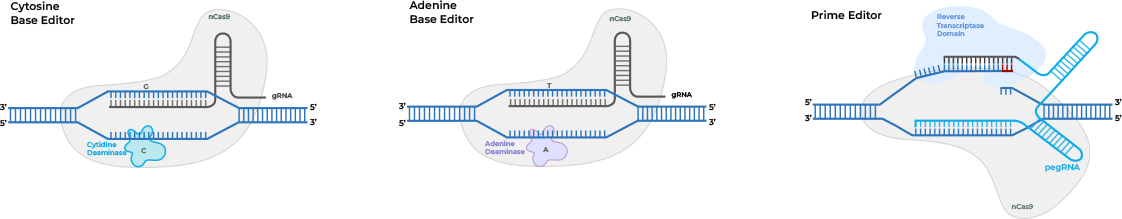
Cas9 nickases also could enable novel single-strand CRISPR gene editing techniques that avoid the potential for unintended genomic changes posed by double-strand DNA breaks. Prime editing utilizes a Cas nickase protein fused to a reverse transcriptase (RT) to write a new sequence into a target DNA site directly. Prime editing guide RNAs (pegRNA) contain both a DNA targeting sequence and an RT template so that the pegRNA-dCas9 nickase complex can identify the target site, cut a single strand, and write the new sequence. Multiple versions of the prime editing system have been developed to improve editing efficiency.
Base editing utilizes a Cas9 nickase, or alternatively a catalytically dead Cas9 (dCas9) which only binds to target DNA without cutting, fused to a nucleobase deaminase enzyme and a DNA glycosylase inhibitor to make targeted point mutations. For example, cytosine base editors (CBEs) and adenine base editors (ABEs), by fusing Cas9 nickase or dCas9 to a cytidine deaminase like APOBEC or adenine DNA deaminases, can convert C to T (or G to A) and A to G (or T to C).
Traditional Gene Knockin
Gene knock-ins have various applications in biotechnology, including disease modeling and gene and cell therapy applications. CRISPR based gene insertion relies on a pathway different than NHEJ, it is called Homology directed repair (HDR) pathway. HDR process is very precise allowing for accurate gene knockin, but HDR occurs in less frequency compared to NHEJ leading to less editing efficiency compared to knockout. KI experiments also require the introduction of a Donor DNA HDR templates, which could be either single- or double-stranded DNA designed with overlapping homology arms to the specific cut site.
To increase the efficiency of HDR, experiment optimization by testing different template format, delivery approaches, culture conditions, as well as using small molecules for cell cycle synchronization, enhance HDR or inhibit NHEJ have been used to overcome this challenge.
Read more on HDR template formats available and design.

CRISPR Transcriptional Regulation
Additional Cas9 variants have been developed for transcriptional regulation of gene expression. Rather than editing DNA, these deactivated Cas9 proteins simply bind the target site and activate or inhibit expression.
CRISPR activation (CRISPRa) systems utilize a dCas9 fused to transcriptional activators to up regulate endogenous gene expression, whereas CRISPR interference (CRISPRi) systems use dCas9 fused to transcriptional repressors to downregulate gene expression. Multiple CRISPRa and CRISPRi systems have been developed using different activators or repressors. CRISPRa gRNAs for the SAM system are currently available in plasmid formats.
Researchers have also recently developed a Cas fusion protein, CRISPRoff, that can epigentically modify and silence genes without editing the underlying genome. The epigenetic memory persists through cell differentiation and is heritably passed to future cell generations. Gene activity can be re-activated using a different fusion protein called CRISPRon.
Technique Components Required Common Experiments Common Applications CRISPRa CRISPRa guide RNA + dCas9-SAM
(or other)Overexpression screening and hit analysis Drug discovery CRISPRi CRISPRi guide RNA + dCas9-KRAB
(or other)Transient loss-of-function, siRNA validation Drug discovery CRISPRoff/
CRISPRonCRISPRoff guide RNA + CRISPRoff Heritable gene silencing without DNA editing Gene therapy 
Subscribe to Receive Updates
& Promotions From GenScript* We'll never share your email address with a third-party.
Latest News & Blogs
Find More CRISPR NewsCRISPR Screening to Optimize CAR Therapies | GenScript

Engineered NK cells, off-the-shelf solution | GenScript

Multiplex Editing T Cells with BE and Cas12 | GenScript

Theory to action: Applying CRISPR in Research

Evolving in vivo Gene and Editing-Cargo Delivery Strategies | GenScript

Enabling Non-Viral T Cell Engineering with ssDNA HDR Templates


-



































