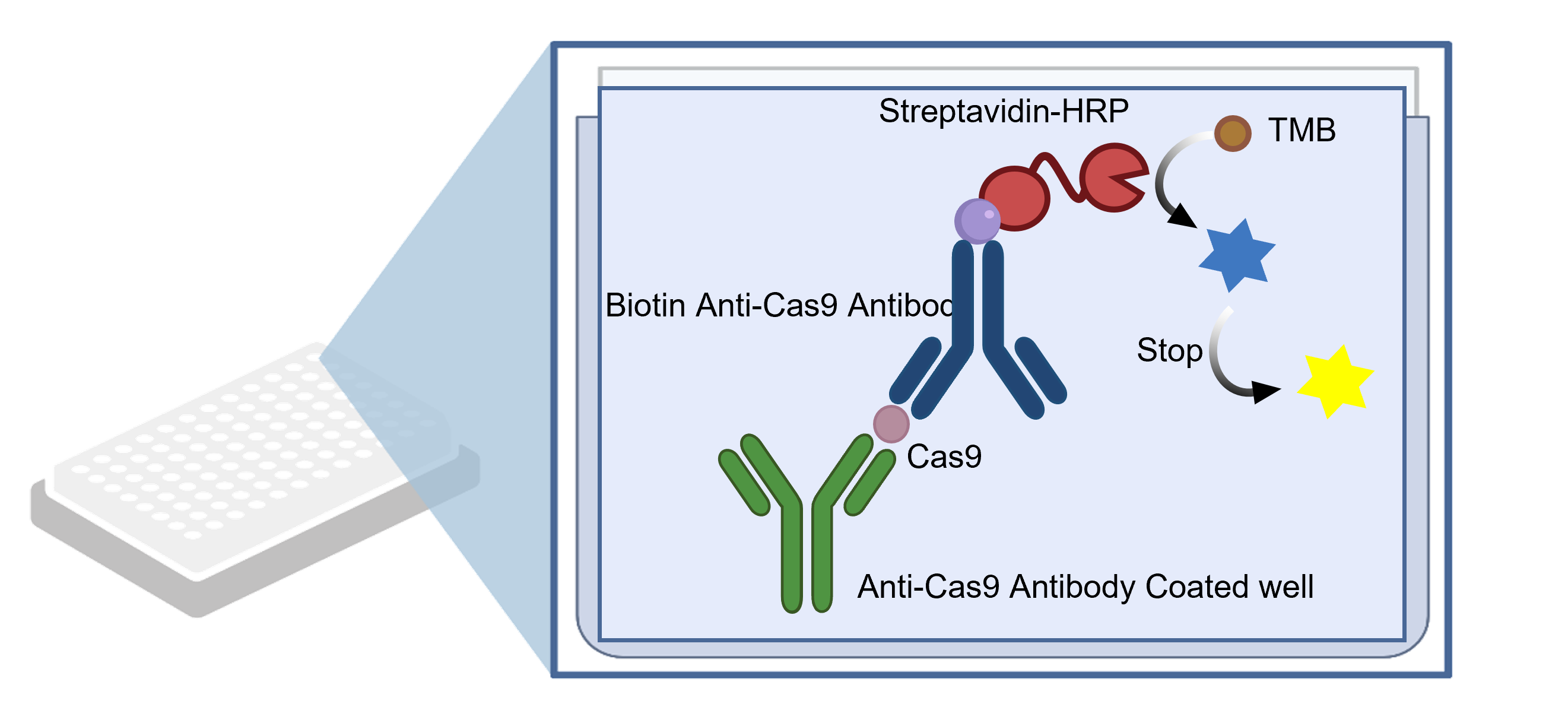
Figure 1. Schematic diagram of Cas9 ELISA kit detection principle
 IVD Raw Materials
IVD Raw Materials

Catalog Products » ELISA Detection Kits » Cas9 ELISA Kit
CRISPR-Cas9 technology is revolutionizing life sciences and propelling the development of next-generation cell therapies. As these groundbreaking treatments progress toward clinical application, ensuring their safety, efficacy, and quality is more important than ever.
One key challenge in non-viral cell therapy platforms using CRISPR-Cas9 is the detection of residual Cas9 protein. Undetected Cas9 residues can compromise both therapeutic stability and patient safety, making accurate quantification a critical step in the development and manufacturing process.
To meet this need, GenScript Biotech has developed a high-sensitivity Cas9 Residual Detection ELISA Kit to support more reliable safety assessment and process monitoring—empowering the future of gene and cell therapies.
of different variants and NLS
(nuclear localization signals)
To as low as 0.125 ng/ml
(10x lower than alternative providers)
No non-specific binding and eliminated background signal
Consistent recovery between 80-120% for various sample amount
Your search returns no product results. Please explore our custom peptide synthesis solutions.

Figure 1. Schematic diagram of Cas9 ELISA kit detection principle
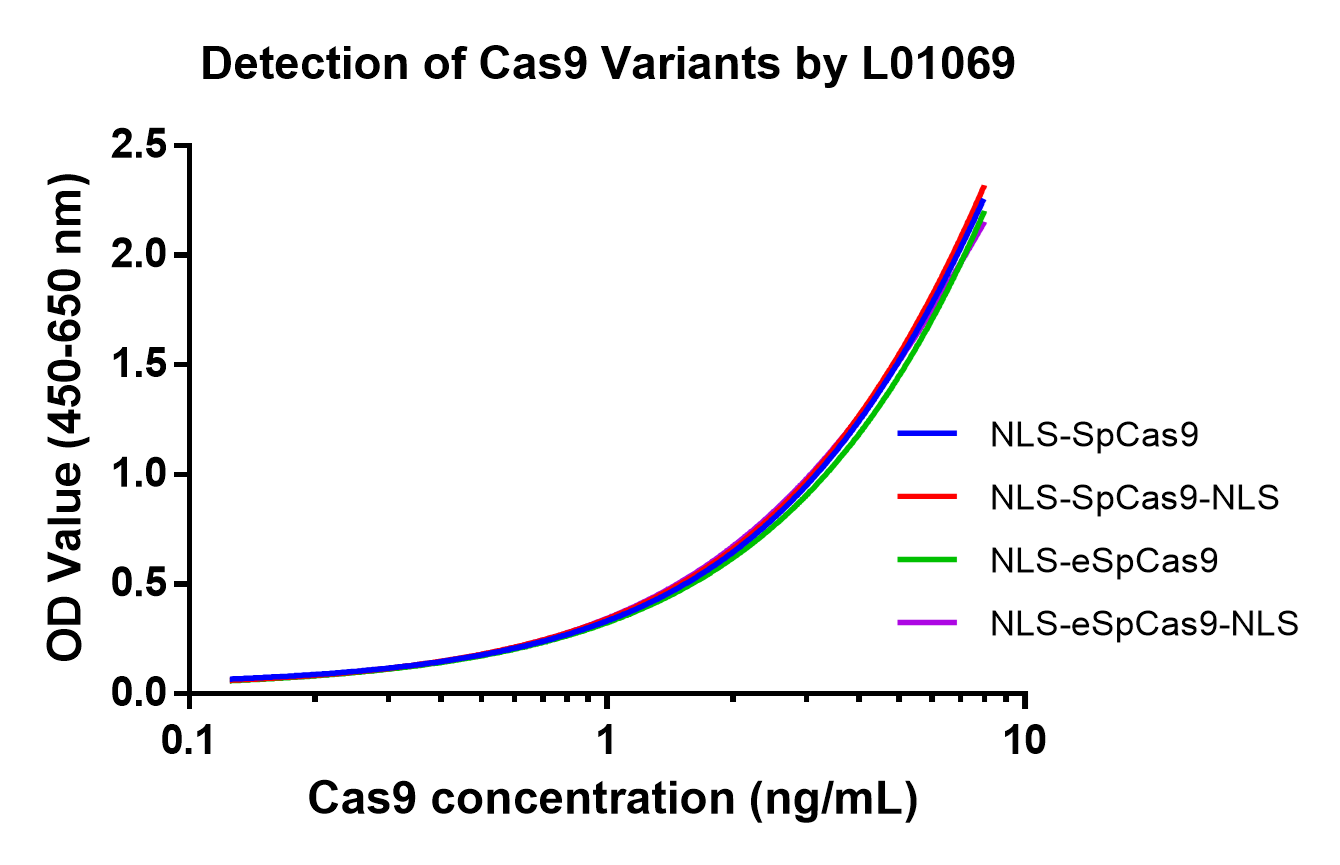
Figure 2. Different Cas9 variants and NLS types/numbers/positions can be detected by Cas9 ELISA Kit (Cat.No. L01069).
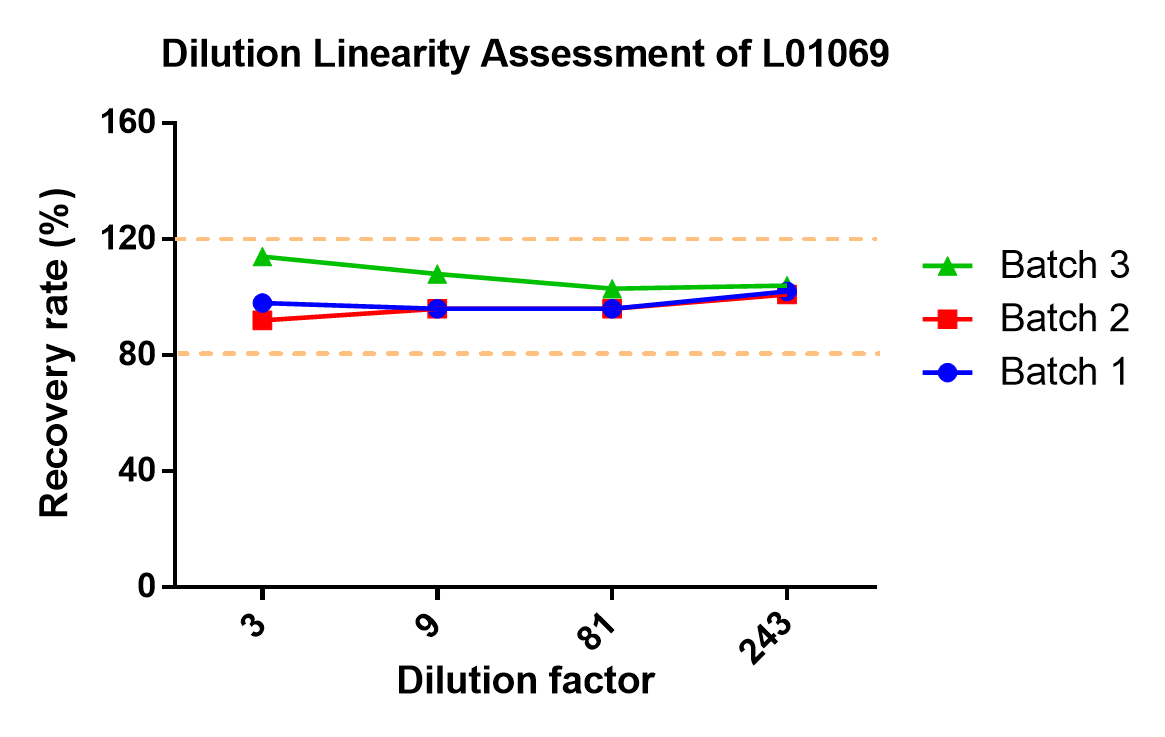
Figure 3. Three batches of Cas9 ELISA kits (Cat.No. L01069) were used to test samples with different dilution factors to evaluate the recovery rate. The result showed that the recovery rates of different batches of kits are not affected by the dilution factor.
The Cas9 ELISA kit (Cat.No. L01069) was used to test different CAR-T samples with different spike concentration (Table 1). The results showed that the kit achieved recovery rate between 80%-120% in all samples (Figure 4), demonstrating its accuracy and repeatability across a range of sample conditions
| Sample ID | Theoretical Spike Conc. (ng/mL) |
Measured Spike Conc. (ng/mL) |
Spike Recovery Rate |
|---|---|---|---|
| Sample 1 | 0.250 | 0.240 | 96% |
| Sample 2 | 0.829 | 0.712 | 87% |
| Sample 3 | 1.000 | 1.071 | 107% |
| Sample 4 | 1.228 | 1.222 | 99% |
| Sample 5 | 1.273 | 1.108 | 87% |
| Sample 6 | 1.419 | 1.324 | 93% |
| Sample 7 | 1.804 | 1.689 | 94% |
| Sample 8 | 2.272 | 2.273 | 100% |
| Sample 9 | 2.379 | 2.230 | 94% |
| Sample 10 | 4.000 | 3.848 | 96% |
Table 1
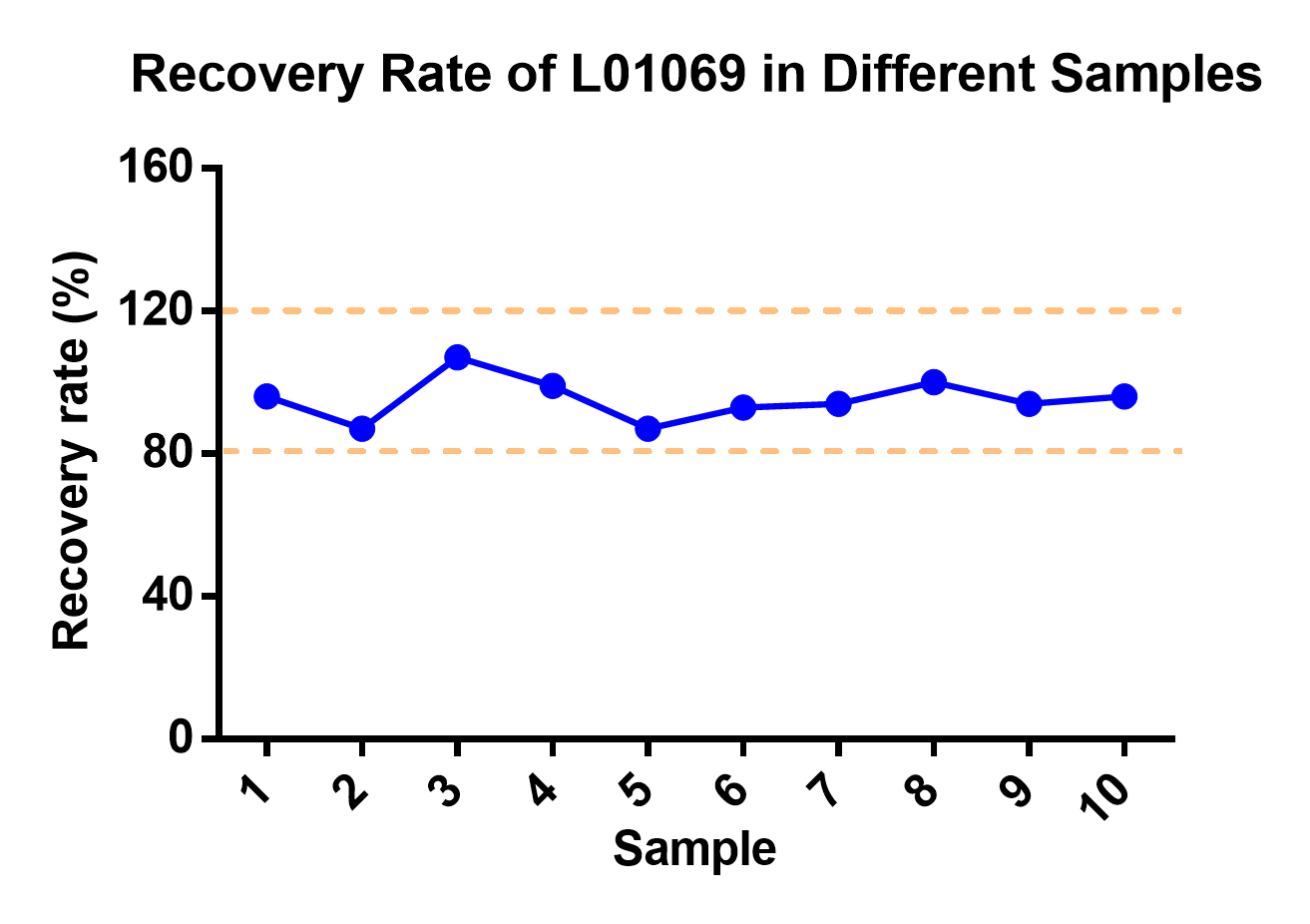
Figure 4
Three different batches of Cas9 ELISA kit were used to test three different samples with variant concentration levels (Table 2). The results showed that the intra-batch and inter-batch CV are both less than 10%, and accuracy above 95% across all conditions (Figure 5).
| Theoretical Conc. (ng/mL) | Intra-assay (n=10) | Inter-assay (n=30) | ||||
|---|---|---|---|---|---|---|
| Average Measured Conc. (ng/mL) | CV | Accuracy | Average Measured Conc. (ng/mL) | CV | Accuracy | |
| 6 | 5.813 | 5.3% | 97% | 5.863 | 7.0% | 98% |
| 1.5 | 1.503 | 4.1% | 100% | 1.471 | 6.6% | 98% |
| 0.375 | 0.383 | 4.9% | 102% | 0.376 | 6.8% | 100% |
Table 2
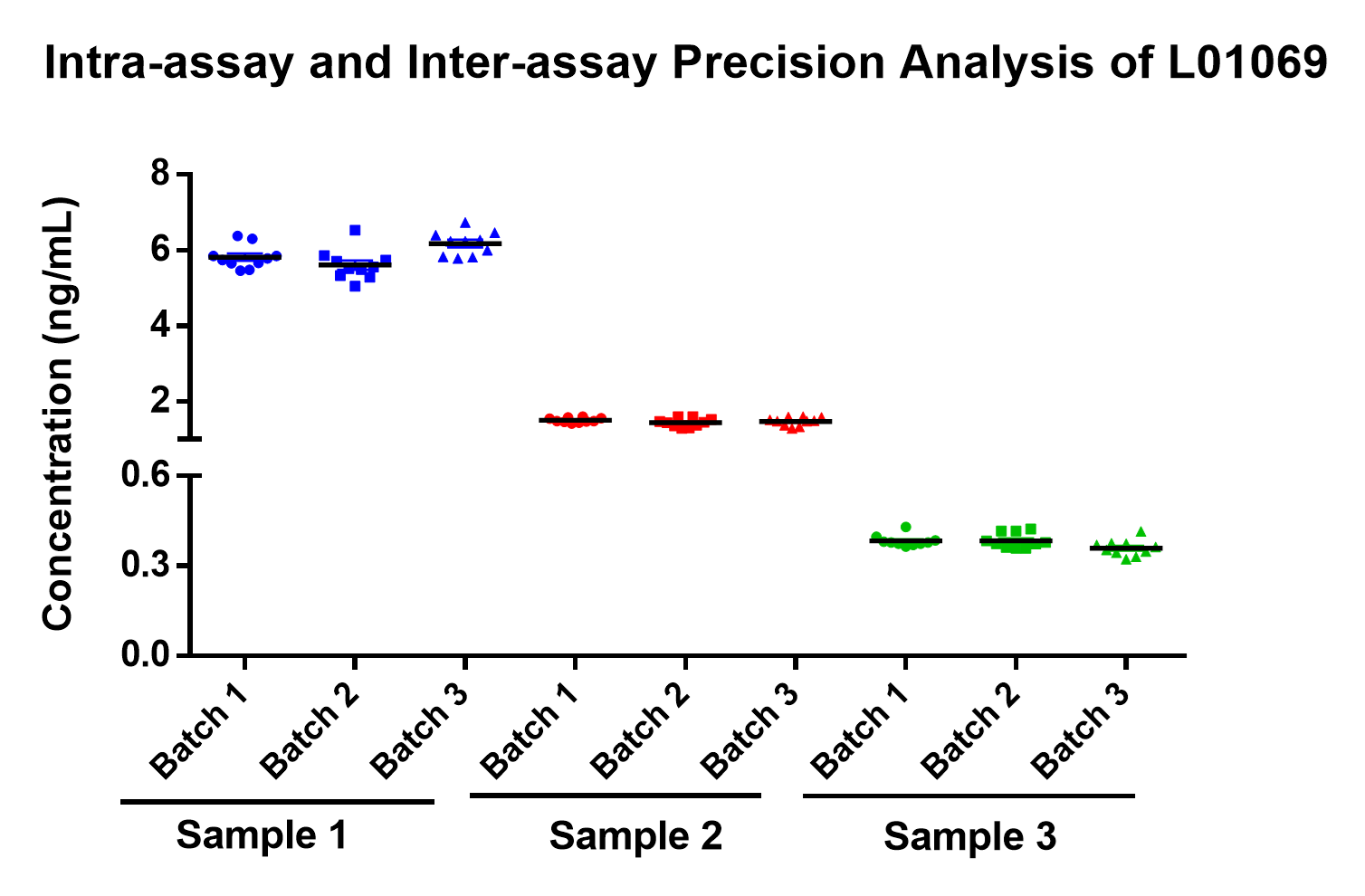
Figure 5
Impurity Test
| Cat.No. | Product Name | Size |
|---|---|---|
| L00976 | BSA ELISA Kit, 2G | 96 Tests |
| L00350 | ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit | 16/32 rxns |
| L00351 | ToxinSensor™ Gel Clot Endotoxin Assay Kit | 1 Kit |
Viral Titration
| Cat.No. | Product Name | Size |
|---|---|---|
| L00938 | Lentivirus Titer p24 ELISA Kit | 96 Tests |
| L00974 | Lentivirus Titer p24 ELISA Kit Pro | 96 Tests |
| L01019 | Lentivirus Titer p24 Kit Easy | 96 Tests |
| L01041 | MuLV Titer p30 ELISA Kit | 96 Tests |
CAR Detection Antibodies
| Product Type | Product Recommendation | Application |
|---|---|---|
| VHH CAR Detection | MonoRab™ Anti-VHH Antibodies | Flow Cytometry |
| scFv CAR Detection | MonoRab™ Anti-scFv antibodies | Flow Cytometry |
| Anti-Tag/Linker Antibody | Epitope Tag Antibody | Flow Cytometry |
| Anti-GS Linker Antibody |