-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Products
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Online Shop
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER
Resources » Weekly Scientific Insight » Therapeutic Antibody Development: Innovations, Trends, and Future Projections
Amanda.Grimm
Therapeutic Antibody Development: Innovations, Trends, and Future Projections
Editor: Amanda GrimmIntroduction
Therapeutic antibodies are antibodies that are used as drugs to treat diseases and are increasingly being used as treatment options for cancer, autoimmune disorders, infectious diseases, and chronic inflammatory diseases1. They perform varying functions such as eliciting molecular pathways and delivering drugs to targeted locations. Some target a single epitope, while others elicit dual pathways through bispecific two-epitope binding, yet others undergo modification with chemical linkers for expert drug delivery. Between June 19, 1986, to December 31, 2023, the FDA approved 145 antibody drugs2 with more approvals rolling in as the current year progresses.
Here, we explore the evolution of therapeutic antibodies, discuss the challenges we face in their development, study the current trends, and take a peek at the exciting future of antibody drugs.
The evolution of antibodies
Kohler and Milner pioneered the monoclonal antibody technology in 19753. Since then, antibody drugs have evolved from mouse-based hybridomas to fully humanized antibodies.
The first US FDA-approved therapeutic antibody, a mouse hybridoma antibody based on Kohler and Milner’s technology, was introduced to the clinic in 19863. This antibody drug is still available today and is used for treating acute tissue rejection by targeting the CD3 receptor on T-cells.
While this technology got us started, mouse-derived therapeutics had some drawbacks. The human immune system can react to antibodies of foreign origin, developing a human anti-mouse antibody (HAMA) response with mild to extreme symptoms. Such reactions can also destroy the mouse antibodies before they have a therapeutic effect3. Patients could also develop mild to severe allergies from these mouse-based antibodies.
By 1997, with daclizumab targeting the IL-2 receptor to prevent transplant rejection, we saw new technologies like humanized antibodies receive US FDA approval. Humanization improved efficacy and safety by engineering strong non-human antibodies with human framework sequences that reduced toxicity while allowing the antibodies to retain their binding activity3.
This success paved the way for exploring antibodies to treat chronic diseases such as cancer and autoimmune diseases. In 2018, Regeneron’s LIBTAYO®4 was approved for treating cutaneous squamous cell carcinoma that had spread to other areas of the body or had not spread beyond the chest but could not be treated by radiation therapy or chemotherapy.
The next big step was the invention and use of nanobodies. Nanobodies are parts of antibodies that can independently bind to specific targets, making them the smallest known antigen-binding entities. Being small, nanobodies stand out for their high solubility, stability, and tissue penetration while causing low immunogenicity. In 2019, Sanofi’s Cablivi®5 became the first FDA-approved Nanobody®-based drug, approved for treating thrombotic thrombocytopenic purpura, a rare blood clotting disorder.
Antibody drugs are also being generated to treat neurodegenerative diseases where previously, the limited treatments available have been to manage symptoms. LEQEMBI®6, developed by Eisai Co., Ltd. and Biogen Inc., was FDA-approved in July 2023 for mild cognitive impairment or mild dementia due to Alzheimer’s disease. Additionally, earlier this month, Eli Lilly’s Kinsunla™7 was FDA-approved to treat early symptomatic Alzheimer’s disease.
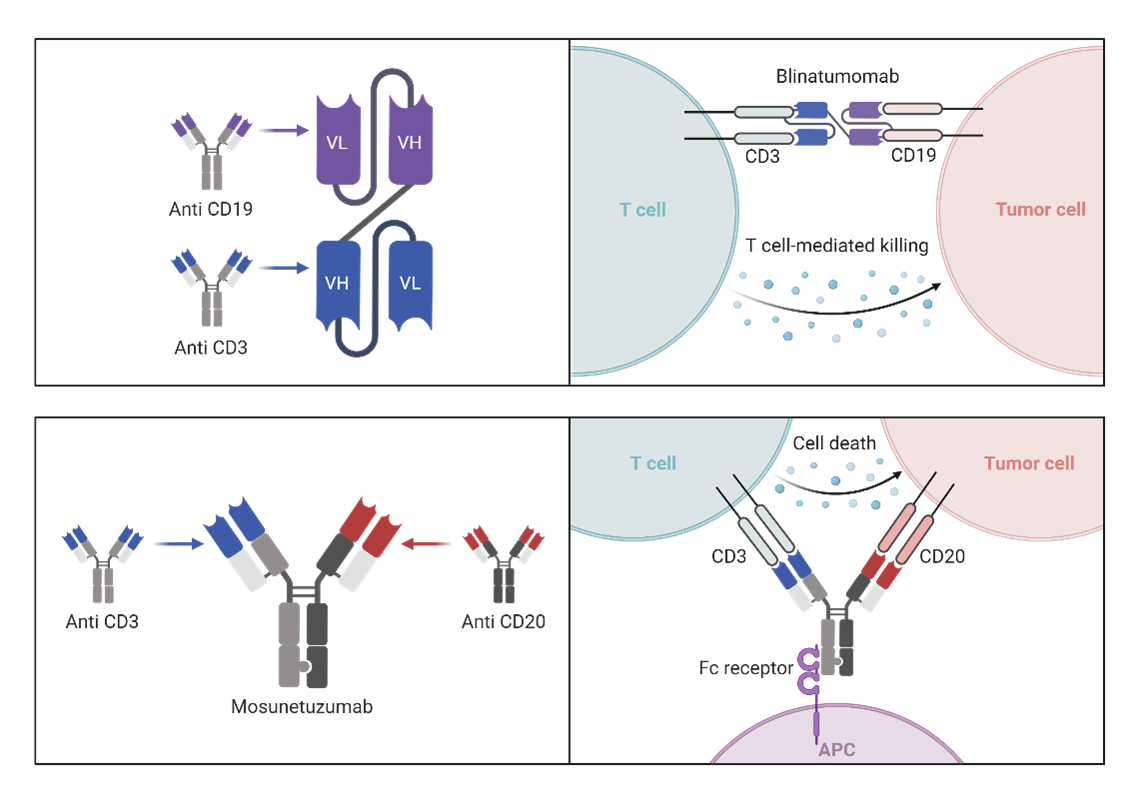
Image source: Biorender.com
Figure 1. Visual representation of different mechanism of action for each bispecific T cell engagers, Blinatumomab (top) and Mosunetuzumab (bottom). Blinatumomab is an approved therapeutic for acute lymphoblastic leukemia that activates T cells by binding CD3 and targets the B cells by binding CD19. Mosunetuzumab is a treatment approved for relapsed or refractory follicular lymphoma that binds CD3 on T cells and CD20 on B cells.
Current trends in personalized antibody therapy and combo treatments
As more and more antibody drugs gain FDA approval, researchers in the field are now exploring how to make antibody-based drugs even more powerful and effective at curing diseases.
Treatment outcomes for any treatment tend to vary from person to person. Scientists are studying what makes a certain treatment effective for some people but ineffective for others. Learning what factors in a person’s body favor certain treatments over others can help generate personalized medicines. Such treatments will be tailor-made for each patient, giving them the best possible chance of recovery.
The effect of sex on treatment outcomes
We are increasingly recognizing that immune responses can be sexually dimorphic8- biological males and females can have different immune responses to the same treatment. Scientists are currently studying why this happens. For example, Dr. Dhifaf Sarhan’s group at Karolinska Institute is studying9:
- Which distinct genes and proteins are expressed in tumors in males vs. females?
- Are there sex-specific soluble biomarkers for pancreatic cancer?
- Can we generate sex-specific antibody treatments to suppress tumors?
In clinical trials10, many cancer treatments have been shown to have sex-specific outcomes. The majority of chemotherapies, antibody therapies, and antibody-drug conjugates (ADCs) appear to provide more benefits to females, though some favor males. Therefore, the best treatment outcomes may depend on assigning male-favoring treatments to males and female-favoring treatments to females.
This sex-based approach to personalized medicine opens new avenues for tailoring antibody treatments to individual patients, potentially leading to more effective therapies and improved outcomes. It also highlights the importance of considering demographic factors in clinical trials and drug development processes.
Theranostics- using diagnostics to tailor therapy
Another critical aspect of personalization in antibody treatments is the integration of diagnostics with therapeutics, often referred to as theranostics. This approach combines diagnostic tests with targeted treatments, allowing for more precise and effective therapies. A prime example is the Bond Oracle HER2 IHC system, which identifies HER-2 protein overexpression in breast tissue samples. This diagnostic tool helps clinicians determine which patients are suitable candidates for treatment with Herceptin (trastuzumab), a monoclonal antibody targeting HER-2-positive breast cancer.
The theranostic approach extends beyond breast cancer, with similar strategies being developed for various cancer types and other diseases. For instance, PD-L1 expression testing is used to identify patients who are more likely to respond to immune checkpoint inhibitors like pembrolizumab or atezolizumab. In the field of hematology, genetic testing for specific mutations can guide the use of targeted antibody therapies in leukemias and lymphomas.
Real-time disease monitoring
The advent of liquid biopsy technologies is further enhancing the potential for personalized antibody treatments. These non-invasive tests can detect circulating tumor DNA or cells, allowing for real-time monitoring of disease progression and treatment response. This information can be used to adjust antibody dosing or switch to alternative therapies more quickly, optimizing treatment outcomes.
Using DNA sequence to tailor treatment
As our understanding of biomarkers and genetic factors influencing drug response grows, we can expect to see an increase in companion diagnostics developed alongside new antibody therapeutics.
Combination therapies
Combination therapies involving antibody drugs have gained significant traction in recent years, particularly in treating cancers and autoimmune diseases. Researchers and clinicians have found that combining antibodies with other therapeutic modalities can enhance efficacy and overcome treatment resistance.
For instance, the combination of immune checkpoint inhibitors like pembrolizumab or nivolumab with targeted antibodies or chemotherapy has shown improved outcomes in various cancer types.
In 2023, the FDA approved the combination of pertuzumab, trastuzumab, and hyaluronidase-zzxf with chemotherapy for HER2-positive breast cancer, demonstrating the potential of antibody-based combination approaches.
For instance, the combination of immune checkpoint inhibitors like pembrolizumab or nivolumab with targeted antibodies or chemotherapy has shown improved outcomes in various cancer types.
Another notable approval was the combination of duvalisib with rituximab for relapsed or refractory chronic lymphocytic leukemia/small lymphocytic lymphoma.
These successes have spurred further research into novel combinations, including dual antibody therapies, ADCs paired with immunotherapies, and antibodies combined with small molecule inhibitors. Clinical trials are ongoing to explore these combinations across a wide range of indications, with the goal to improve patient outcomes and address unmet medical needs. As our understanding of disease mechanisms and drug interactions deepens, we can expect to see more innovative and effective antibody-based combination therapies reaching the market in the coming years.
Global trends in development and market growth
Over time, antibody drugs have gained so much popularity that they are now the best-selling drugs3 in the pharmaceutical market. The global market size of antibody drugs was US $200.18 B in 2022 and is expected to reach a staggering $605.8 B by 203211. In 2021, 6 of the 10 top-selling prescription drugs12 in the US were biologics. Ahead, we highlight several factors driving the robust growth in the antibody drug market.
Bispecific antibodies and ADCs have emerged as particularly promising formats, attracting significant attention at industry conferences and substantial investment from pharmaceutical companies. This trend is reflected in the numerous collaborations and acquisitions between large pharmaceutical companies and specialized antibody discovery companies.
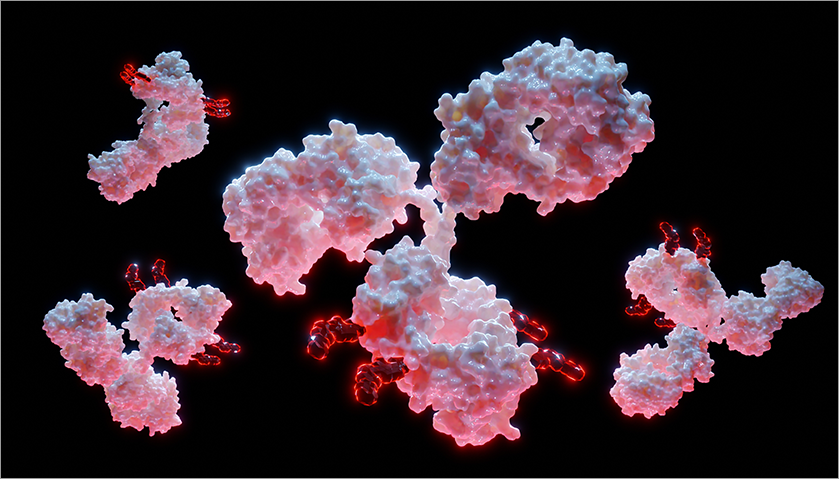
Image source: Shutterstock
Figure 2. Visual example of an antibody drug conjugate (ADC) including antibody (pink) and linker + drug (red).
The market is further bolstered by the expanding applications of antibodies across various disease areas, including oncology, autoimmune disorders, and infectious diseases. Emerging markets, particularly in Asia, are contributing to this growth as the healthcare infrastructure improves and access to advanced therapies increases.
Additionally, the integration of AI and machine learning in antibody discovery and development is accelerating the pipeline and reducing costs, potentially leading to more diverse and affordable antibody therapies.
As personalized medicine gains traction, the demand for targeted antibody therapies is expected to rise, further driving market expansion. These factors collectively point to a continued upward trajectory for the global antibody therapeutics market in the coming years.
Challenges and considerations in antibody development
There are several challenges that need to be overcome when developing therapeutic antibodies.
Many antibody candidates are first screened using in vitro studies, and the antibodies that perform well are chosen for development. At this stage, factors that affect how well an antibody can be developed at scale determine its potential as a drug13. Developability is assessed by analyzing the antibody’s protein sequence, 3D structure, and molecular dynamics13. The earlier this can be confirmed, the sooner the developer can determine if the antibody candidate is worth pursuing in more expensive studies.
Complex antibodies like multi-specifics or ADCs also require careful design and production. The drug needs to have a proven function for all the intended targets. ADCs, in particular, are generated considering multiple parameters such as the antibody format, linker type, and the amount of drug that can successfully be attached. Once complete, researchers ensure the drug is delivered successfully, and it can effectively bind the target.
How GenScript helps researchers overcome challenges of antibody generation
Developers want to iterate quickly. CROs such as GenScript mobilize to offer expedited turn-around times for the various services critical for antibody discovery, including molecular biology, protein expression, and antibody development. There are many new and notable options available to researchers.
- The proprietary and scalablea TurboCHO™ recombinant protein and antibody expression platform includes an expression option at each stage of development. The newly upgraded 2.0 version incorporates AI-powered codon optimization, updated processes to mitigate mistakes, stringent QC, and IP protection.
- TurboCHO™ is also the key support platform for bispecific antibody-focused design and expression. The platform allows researchers to choose from a range of format options for their bispecific antibodies and helps them assess developability capabilities.
- The new FLASH Gene service offers next-level discovery options bundling simple gene synthesis with cloning and plasmid prep at an affordable flat rate completed in the market’s fastest turnaround time. Developers can screen more possibilities and identify the best antibody candidates faster when using this service.
1 CrossMabCH1-CL
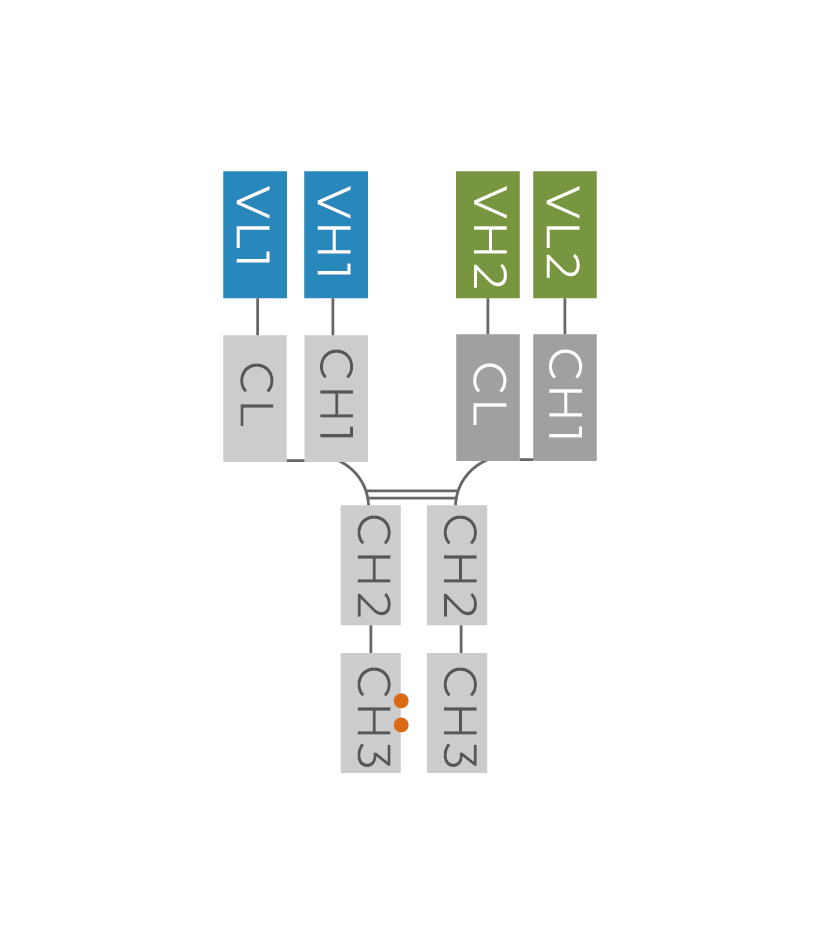
Manufacturability Score 1-5 according to bsAb’s expression level and purification difficulty defined by GenScript
(e.g. score 1 represents the most difficult)2 Recommend purification step(s) 2 steps Typical yield Low: <50mg /L
Moderate: 50 – 200mg/L
High: >200mg/LHigh Image source: GenScript
Figure 3. Bispecific antibody example from our Interactive Production Map.
Use of AI to optimize workflow
Advancements in artificial intelligence and machine learning are creating new opportunities for generative, modeling, and predictive platforms. These new platforms open doors to new drug design possibilities while accelerating the design and screening processes14.
Biopharma Dealmakers highlights3 major collaborations between key pharma leaders AbbVie, AstraZeneca, and Sanofi with AI experts BigHat Biosciences, Absci, and BioMap, respectively.
GenScript continues to incorporate its own AI into many of the available services. A key implementation is its codon optimization tool- whether customers take advantage of the GenSmart™ platform on their own or through one of the protein and antibody expression services. Sequence analysis and codon optimization are standard features of the design process that ensure proper protein expression and antibody generation. Additionally, developability assessment has been integrated into the TurboCHO™ SmartQuote quote and purchase process. This newest feature provides multiple prediction factors like post-translational modification, aggregation sites, degradation sites, immunogenicity, and viscosity provided in an easy-to-use annotated report.
Looking to the future
The field of therapeutic antibodies has come a long way since the first FDA approval in 1986. From mouse-derived antibodies to fully humanized versions and now to complex bispecific antibodies and ADCs, the evolution has been remarkable. The integration of cutting-edge technologies such as artificial intelligence and machine learning is further accelerating antibody discovery and development processes.
As we look to the future, several trends are shaping the landscape of antibody therapeutics:
- Personalized medicine is gaining traction, with researchers exploring how factors like a person’s sex can influence drug efficacy.
- Combination therapies are showing promise in enhancing treatment outcomes.
- The COVID-19 pandemic has renewed interest in antibodies for infectious diseases.
- Bispecific antibodies and ADCs are at the forefront of innovation, attracting significant attention and investment.
- Global collaborations between large pharmaceutical companies and specialized antibody discovery firms are driving progress in the field.
While challenges remain, particularly in developability and complex antibody design, the future of antibody therapeutics looks bright. With continued advancements in technology and our understanding of disease mechanisms, antibodies are poised to play an even more crucial role in treating a wide range of conditions, as we see with cancer and autoimmune diseases. As the field evolves, we can expect to see more targeted, effective, and personalized antibody-based treatments that will significantly impact patient care and outcomes.
References
-
1. CDER. (2024). Bispecific Antibodies: An Area of Research and Clinical Applications. FDA. https://www.fda.gov/drugs/spotlight-cder-science/bispecific-antibodies-area-research-and-clinical-applications
-
2. Strohl WR. Structure and function of therapeutic antibodies approved by the US FDA in 2023. Antibody therapeutics. 2024;7(2):132-156. doi:https://doi.org/10.1093/abt/tbae007
-
3. Lu RM, Hwang YC, Liu I-Ju, et al. Development of Therapeutic Antibodies for the Treatment of Diseases. Journal of Biomedical Science. 2020;27(1):1-30. doi:https://doi.org/10.1186/s12929-019-0592-z
-
4. LIBTAYO® is a registered trademark of Regeneron Pharmaceuticals Inc. and Sanofi, for more information visit: FDA Approves Libtayo® (cemiplimab-rwlc) as First and Only Treatment for Advanced Cutaneous Squamous Cell Carcinoma | Regeneron Pharmaceuticals Inc.
-
5. Cablivi® is a registered trademark of Sanofi, for more information visit: https://www.sanofi.com/en/media-room/press-releases/2019/2019-02-06-16-43-21-1711610
-
6. LEQEMBI® is a registered trademark of Eisai Co., Ltd., and Biogen Inc., for more information visit: https://media-us.eisai.com/2023-07-06-FDA-Grants-Traditional-Approval-for-LEQEMBI-R-lecanemab-irmb-for-the-Treatment-of-Alzheimers-Disease
-
7. Kisunla™ is trademarked by Eli Lilly and Company, for more information visit: https://investor.lilly.com/news-releases/news-release-details/lillys-kisunlatm-donanemab-azbt-approved-fda-treatment-early#:~:text=INDIANAPOLIS%20%2C%20July%202%2C%202024%20%2F,(AD)%2C%20which%20includes%20people
-
8. Capone I, Marchetti P, Ascierto PA, Malorni W, Gabriele L. Sexual Dimorphism of Immune Responses: A New Perspective in Cancer Immunotherapy. Frontiers in Immunology. 2018;9. doi:https://doi.org/10.3389/fimmu.2018.00552
-
9. Research line I: Studies of sexual immune dimorphism in the TME | Karolinska Institutet. ki.se. Accessed July 14, 2024. https://ki.se/en/labmed/research/research-group-leaders-at-labmed-a-o/tumor-immunology-immunotherapy-group/research-line-i-studies-of-sexual-immune-dimorphism-in-the-tme
-
10. Kammula AV, Schäffer AA, Rajagopal PS, Kurzrock R, Ruppin E. Outcome differences by sex in oncology clinical trials. Nature Communications. 2024;15(1):2608. doi:https://doi.org/10.1038/s41467-024-46945-x
-
11. Antibodies Drug Market Size Envisioned at USD 605.8 Billion by 2032. www.towardshealthcare.com.
-
12. Horrow C, Gabriele SME, Tu SS, Sarpatwari A, Kesselheim AS. Patent Portfolios Protecting 10 Top-Selling Prescription Drugs. JAMA Internal Medicine. Published online May 13, 2024. doi:https://doi.org/10.1001/jamainternmed.2024.0836
-
13. Bauer J, Rajagopal N, Gupta P, Gupta P, Nixon AE, Kumar S. How can we discover developable antibody-based biotherapeutics? Frontiers in Molecular Biosciences. 2023;10:1221626. doi:https://doi.org/10.3389/fmolb.2023.1221626
-
14. Dealmakers B. Antibody design enters the AI era. Biopharma Dealmakers. Published online May 31, 2024. doi:https://doi.org/10.1038/d43747-024-00030-w
Subscribe to have the latest weekly scientific insights delivery to your inbox!
* We'll never share your email address with a third-party.

-








































