-
REAGENT SERVICES
Hot!
-
Most Popular Services
-
Molecular Biology
-
Recombinant Antibody/Protein
-
Reagent Antibody
-
CRISPR Gene Editing
-
DNA Mutant Library
-
IVT RNA and LNP Formulations
-
Oligo Synthesis
-
Peptides
-
Cell Engineering
-
- CRISPR/Cas9 sgRNA
- CRISPR/Cas12a crRNA
- Prime Editing Guide RNA
- Base Editing Guide RNA
- HDR Templates
- gRNA + HDR Template Design Tools
- cGMP Guide RNA
- cGMP HDR Templates
- CRISPR/Cas Proteins
- CAR-T Knock-in Optimization Kit
- CRISPR Plasmids
- CRISPR gRNA Plasmid Libraries
- CRISPR Cell Lines
- Microbial Genome Editing
-
-
PRODUCTS
-
Most Popular Products
-
Antibodies
-
ELISA Kits
-
Protein Electrophoresis and Blotting
-
Protein and Antibody Purification
-
Recombinant Proteins
-
Molecular Biology
-
Stable Cell Lines
-
Cell Isolation and Activation
-
 IVD Raw Materials
IVD Raw Materials
-
 Therapy Applications
Therapy Applications
-
Online Shop
-
Resources
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
![Anti-Camelid VHH]() MonoRab™ Anti-VHH Antibodies
MonoRab™ Anti-VHH Antibodies
-
![Precast Gels]() SurePAGE™ Precast Gels
SurePAGE™ Precast Gels
-
![Quatro ProAb Automated Protein and Antibody Purification System]() AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
AmMag™ Quatro ProAb Automated Protein and Antibody Purification System
-
![Target Proteins]() Target Proteins
Target Proteins
-
![AmMag™ Quatro Automated Plasmid Purification]() AmMag™ Quatro automated plasmid purification
AmMag™ Quatro automated plasmid purification
-
 IVD Raw Materials
IVD Raw Materials
-
![Quick
Order]() Quick Order
Quick Order
-
![Quick
Order]() Quick Order
Quick Order
- APPLICATIONS
- RESOURCES
- ABOUT US
- SIGN IN My Account SIGN OUT
- REGISTER

![Antibody Basics Antibody Basics]()
Antibody Basics
Resources » Technical Resource Centers » Antibody Drug Discovery Technical Resource » Antibody Basics
- The History of Antibodies
- Antibody Basics
- Antibody Formats
- Antibody Drug Discovery Overview
- Target Identification and Validation
- Antibody Drug Discovery Webinars
- Protein Webinars
- Hit Generation and Screening
- Lead Selection and Optimization
- Candidate Selection
- Antibody Expression in E.coli
- Transient vs. Stable Expression
- Antibody Expression in Eukaryotic Hosts
Overview
Humans have 5 classes of Abs (interchangeably used with Immunoglobulins or Igs). IgG, IgA, IgD, IgE and IgM. All 5 classes are secreted by activated B cells as glycoproteins. All human Igs possess a basic monomeric “H2L2” structure consisting of 2 Heavy (H) chains and 2 Light (L) chains. Each H chain is paired with one L chain. H chains define the class of Ig [symbolized by Greek letter γ, α, δ, ε, μ] and the L chains are comprised of either κ or λ isoforms. Each Ig possesses 2 defined regions. The upper-half antigen-binding regions (Fabs) and the lower-half crystallizable fragment (Fc). The Fc region is composed entirely of H chain whereas the Fab region consists of both H & L chain domains. In IgG, IgA, IgD classes the Fab is separated from the Fc by a flexible hinge region whereas in the IgE and IgM classes an extra constant domain replaces the hinge resulting in less flexibility of the Fab domains. Additionally, IgA and IgM possess the following.
- Tailpiece region that confers ability to form multimers1
- J (Joining) chain region that confers ability to bind specific receptors
Antibody production in the body begins by the expression of IgM and IgD on the surface of naïve B cells in response to antigenic stimuli. Through the process of hyper mutation and class switching, high affinity IgGs are produced. Human IgG is further subdivided into IgG1, IgG2, IgG3 and IgG4 isotypes2.
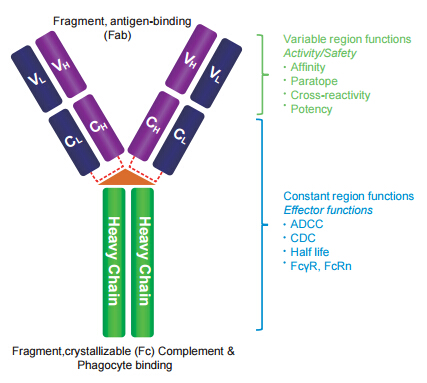
Figure 1: Basic Ab [IgG] structure - Ab is a Y shaped molecule consisting of 2 heavy (H) chains and 2 light (L) chains folded into constant (C) and variable (V) domains. The Fab domain consists of 2 variable and 2 constant domains with the 2 variable domains making up the variable fragment (Fv). The Fv provides antigen specificity of the Ab with the constant domains providing a structural framework. Each Fv contains 3 hypervariable loops known as Complementarity Determining Regions (CDRs). It is the hyper variability of the CDRs that allow an Ab, in theory, to recognize an unlimited number of Antigens. Approximate L chain amino acid residues are 220 and MW is ~25 kDa. Approximate H chain amino acid residues are 455 and MW is ~50 kDa. One L&H chain equals ~75 kDa, thus full length IgG with both L&H chains is ~150 kDa.
Since a vast majority of all therapeutic antibodies employ an IgG1 isotype, it has become the default isotype for many Ab candidates. And hence, more is known about the biology and functionality of IgG1 than any other isotype.
IgG Overview
- Divalent Abs derived from gamma gene locus
- There are 4 isotypes (IgG1, 2, 3, 4)
- Half-life of IgG 1,2 and 4 is 2-3 weeks
- IgG3 half-life is 1 week
- IgGs are the most prevalent Abs found in serum
- Approximate MW is 150 kDa
GenScript Antibody Drug Discovery Services
Antibody Discovery
GenScript’s Antibody Engineering group can build antibody library with up to 1010 individual clones, to speed up your antibody discovery efforts.
Antibody Sequencing
GenScript’s advanced Antibody Sequencing technology offers fast and professional sequencing services for your monoclonal antibodies.
Assays
GenScript has developed several cell-based ADCC/CDC functional assays to profile the efficacy and potency of your therapeutic antibodies using proprietary recombinant effector cells.
Antibody Engineering
GenScript scientists’ extensive experience in antibody engineering can provide superior services such as antibody humanization, affinity maturation and more.
Antibody Production
With solid expertise in recombinant antibody (rAb) production techniques, GenScript provides a comprehensive rAb service portfolio that deliver microgram to gram quantities of pure rAb for each stage of your Ab drug discovery program.
PK/PD Study
GenScript offers over 120 tumor and inflammation models for evaluation of in vivo efficacy, PK/PD, biomarker and bioanalysis studies. GenScript Anti-idiotype Antibody services are also a powerful tool for antibody drug PK/PD and immunogenicity studies.
References
- Sorensen, V., Sundvold, V., Michaelsen, T. E. & Sandlie, I. Polymerization of IgA and IgM: roles of Cys309/Cys414 and the secretory tailpiece. J Immunol 162, 3448-3455 (1999).
- Brandtzaeg, P. & Prydz, H. Direct evidence for an integrated function of J chain and secretory component in epithelial transport of immunoglobulins. Nature 311, 71-73 (1984).
Quotations and Ordering
Click the quote button, provide service information and submitOur Technical Account Manager will contact you within 24 hours to finalize quoteYou review anc approve final order details and priceYou provide credit card/PO information and order is placedProject is initiated immediatelyOur Project Manager will be in contact with you to provide updates
-


































