
![GenXceed Cell-free mRNA Template GenXceed Cell-free mRNA Template]()
GenXceed Cell-free mRNA Template
Beyond Plasmids. Beyond Limits. RCA-Powered DNA for IVT Precision.
- Step 1: Optimized DNA Input for RCA → Proprietary strains and vectors ensure high Poly(A) integrity and sequence stability, optimizing RCA amplification efficiency.
- Step 2: RCA Amplification → Plasmid-Free, exponentially scalable DNA synthesis with zero bacterial contaminants.
- Step 3: Linearization → 99.9%+ DNA purity, 100% plasmid yield, and zero host-cell contaminants.
- Step 4: DNA Purification → Streamlined process, ensures ultra-low endotoxin & IVT compatibility.
- Step 5: Final Purification & QC → Poly(A) integrity verified, with high uniformity.
- Step 6: IVT-Ready DNA → Optimized for Seamless mRNA Synthesis, No Extra Steps Needed.
Competitive Advantages
|
Process Step |
|
GenXceed™ Cell-Free (RCA-Based) |
|
Traditional Plasmid-Based DNA |
|
|
Amplification |
|
RCA-powered, ultra-fast expansion |
|
Slow bacterial culture & plasmid replication |
|
|
Purification |
|
Streamlined purification, eliminating host DNA |
|
Multi-step plasmid extraction (alkaline lysis, multiple washes, endotoxin removal, etc.) |
|
|
Linearization |
|
Enzymatic digestion for high-fidelity linearization |
|
Additional restriction enzyme digestion required |
|
|
Endotoxin Contamination Risk |
|
<0.05 EU/µg, minimal endotoxin, no bacterial culture |
|
High endotoxin, requires extensive removal |
|
|
Host DNA Contaminants |
|
Effectively minimized, >99% removal of host genomic DNA |
|
Possible bacterial genome contamination, about 15% |
|
|
IVT Readiness |
|
Directly IVT-compatible, Poly(A) Guaranteed |
|
May require further purification & quality control |
|
|
Turnaround Time |
|
As fast as 8 business days |
|
17-22 business days |
|
Why GenXceed™?
RCA-Powered Speed Redefines Industry Standards
High-yield DNA synthesis in just 8 BDs, no large-scale plasmid expansion.
Poly(A) Guaranteed
Verified integrity for peak IVT efficiency—no uncertainties.
Purity Beyond Limits
Minimal host DNA, ultra-low endotoxin, and enzymatic precision for flawless templates.
Next-Gen Disruption
No plasmid baggage, no fermentation—just scalable, high-yield DNA redefined.
Unmatched Purity – Optimized for High-Performance IVT
GenXceed™ delivers ultra-pure, IVT-ready DNA with stringent quality control, ensuring optimal performance in mRNA synthesis. As shown below, our templates exhibit:
- Exceptional DNA Purity – Verified by AGE and IEC-HPLC analysis, achieving >98% purity, minimizing unwanted contaminants.
- Ultra-Low Endotoxin & Contaminants – Ensuring a clean template for high-efficiency IVT.
- Poly(A) Integrity Verified – Sanger sequencing confirms a complete and stable Poly(A) tail, essential for translation efficiency and mRNA stability.
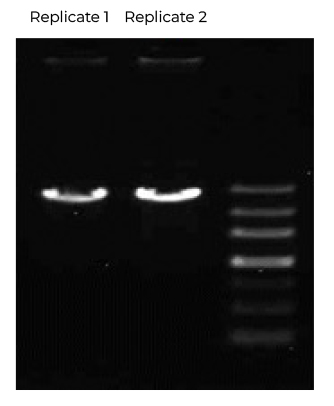
Fig 1. DNA Template Purity by AGE
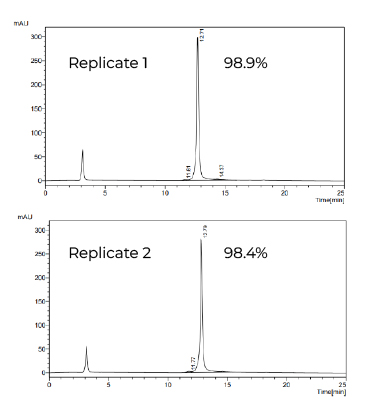
Fig 2. DNA Template Purity by IEC-HPLC

Fig 3. PolyA Uniformity by FA5200 Analyzer
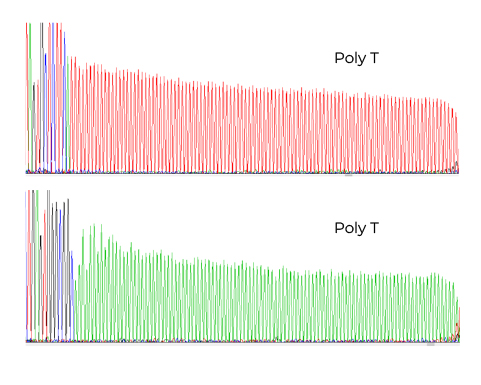
Fig 4. Sanger Sequencing for Poly(A) Validation
Consistent IVT Performance
GenXceed™ mRNA templates deliver unmatched IVT performance—exceptional purity, minimal contaminants, and consistently high expression levels. Engineered for efficiency, our RCA-powered DNA templates ensure seamless translation into reliable, high-quality mRNA, driving superior downstream results.
- IVT mRNA Purity by AGE - Gel electrophoresis confirms the high integrity of IVT mRNA, with no visible degradation or unwanted byproducts.
- Ultra-Low Endotoxin & Contaminants – Ensuring a clean template for high-efficiency IVT.
- Poly(A) Integrity Verified – Sanger sequencing confirms a complete and stable Poly(A) tail, essential for translation efficiency and mRNA stability.
Ditch the delays. Eliminate contamination. Scale with confidence.
GenXceed™ delivers next-generation, IVT-ready DNA in just 8 business days.
Get Started with GenXceed™ Today!
FAQ
-
What makes GenXceed™ Cell-Free mRNA Template different from traditional plasmid-based DNA production?
GenXceed™ utilizes Rolling Circle Amplification (RCA), a cell-free, enzymatic amplification process that eliminates the need for large-scale bacterial culture and plasmid extraction. This results in:
- Higher purity – No bacterial host DNA contamination
- Minimal endotoxin levels – Avoids endotoxin accumulation from bacterial fermentation
- Faster turnaround – As fast as 8 business days, compared to 17-22 days for plasmid-based methods
-
How does GenXceed™ ensure high purity and IVT compatibility?
GenXceed™ templates undergo rigorous multi-step purification and quality control, ensuring:
- Endotoxin levels <0.05 EU/µg – Minimizing immune response risks in downstream applications
- No host DNA carryover – Eliminating bacterial genome contamination
- Pre-validated IVT compatibility – Delivering high-yield, clean transcription
-
How does GenXceed™ linear DNA compare to traditional linearized plasmid DNA?
GenXceed™ RCA-based linear DNA offers superior IVT performance while eliminating key drawbacks of plasmid-derived templates:
- Pre-validated Poly(A) integrity – Ensuring stable and efficient mRNA synthesis
-
Can GenXceed™ support customized mRNA template designs?
Absolutely. Our platform offers flexible design options, including:
- Custom Poly(A) Tail Lengths – Tailored to enhance IVT efficiency for specific applications
- Vector Optimization – Ensuring the highest yield and stability for mRNA synthesis
- Sequence Modifications – Supporting specialized research and therapeutic needs
Get in Touch With GenScript
Cell Free mRNA Template




















































